Knowing the subshells of an electron shell
... Knowing the subshells of an electron shell Fill in the information missing from this table: Some electron shells shell subshells ...
... Knowing the subshells of an electron shell Fill in the information missing from this table: Some electron shells shell subshells ...
Electricity Notes, Part I
... ___________________ charge, when rubbed against other materials. Materials such as wool and hair tend to lose electrons, acquiring a _________________ charge. 6. The unit for electrical potential or potential difference is the ___________. This can be thought of as how much each electron is pushed u ...
... ___________________ charge, when rubbed against other materials. Materials such as wool and hair tend to lose electrons, acquiring a _________________ charge. 6. The unit for electrical potential or potential difference is the ___________. This can be thought of as how much each electron is pushed u ...
SET 2 Option J — Particle physics J1. This question is about
... The quark content of the particles involved is ssd , 0 uds and du . State and explain whether the interaction involved in this decay is electromagnetic, strong or weak. ...
... The quark content of the particles involved is ssd , 0 uds and du . State and explain whether the interaction involved in this decay is electromagnetic, strong or weak. ...
lecture notes, page 2
... • Similar to the angular momentum quantum number, l, ms describes the magnitude of an angular momentum. However, ms completes the description of an __________________ and is NOT dependent on the orbital. The property of electron spin was first proposed by S. Goudsmit and G. Uhlenbeck in 1925 to exp ...
... • Similar to the angular momentum quantum number, l, ms describes the magnitude of an angular momentum. However, ms completes the description of an __________________ and is NOT dependent on the orbital. The property of electron spin was first proposed by S. Goudsmit and G. Uhlenbeck in 1925 to exp ...
Unit 2 note
... Rutherford’s orbital atomic model could not explain the chemical properties of elements. For example, why do metal elements, or compounds that contain metals, give off characteristic colours when heated in a flame? Explaining what leads to the chemical properties of elements requires a model that be ...
... Rutherford’s orbital atomic model could not explain the chemical properties of elements. For example, why do metal elements, or compounds that contain metals, give off characteristic colours when heated in a flame? Explaining what leads to the chemical properties of elements requires a model that be ...
Simple Models for Classical Electron Radius and Spin
... Smallest values for radius are obtained from the electrostatic energy considerations, however these considerations are resulted in very high vr /c ratio. For the smallest radius value a = 3.534 × 10−16 m, the biggest vr /c ratio obtained as 947. This is really huge number for vr /c ratio, and none o ...
... Smallest values for radius are obtained from the electrostatic energy considerations, however these considerations are resulted in very high vr /c ratio. For the smallest radius value a = 3.534 × 10−16 m, the biggest vr /c ratio obtained as 947. This is really huge number for vr /c ratio, and none o ...
Electricity - Effingham County Schools
... Conservation of Charge • According to the law of conservation of charge, charge can be transferred from object to object, but it cannot be created or destroyed. • Whenever an object becomes charged, electric charges have moved from one place to another. ...
... Conservation of Charge • According to the law of conservation of charge, charge can be transferred from object to object, but it cannot be created or destroyed. • Whenever an object becomes charged, electric charges have moved from one place to another. ...
Classical Models of Subatomic Particles
... distances from the particle much larger than the Compton wavelength. The interior solution will be modelled by a quantum distribution. But, however large the matching radius (rM ) is taken to be, the act of measuring the spacetime curvature on a surface at that distance (i.e. measuring the paramete ...
... distances from the particle much larger than the Compton wavelength. The interior solution will be modelled by a quantum distribution. But, however large the matching radius (rM ) is taken to be, the act of measuring the spacetime curvature on a surface at that distance (i.e. measuring the paramete ...
What`s This Thing Called “Current”?
... industry. His equations (known, of course, as Maxwell's Equations) have endured for 130 years and are as valid today as when they were first published2. However, it should be noted that Maxwell was a mathematician, not a physicist. The usual expression of Maxwell's equations encompasses a set of fou ...
... industry. His equations (known, of course, as Maxwell's Equations) have endured for 130 years and are as valid today as when they were first published2. However, it should be noted that Maxwell was a mathematician, not a physicist. The usual expression of Maxwell's equations encompasses a set of fou ...
Unit 3 Notes - WordPress.com
... a. A maximum of _________ electrons can fit in a single orbital. 3. Main energy levels indicate the general amount of __________________ and ___________________ from the nucleus a given electron in an orbital possesses. a. Each ______________, or period, on the periodic table indicates a main energy ...
... a. A maximum of _________ electrons can fit in a single orbital. 3. Main energy levels indicate the general amount of __________________ and ___________________ from the nucleus a given electron in an orbital possesses. a. Each ______________, or period, on the periodic table indicates a main energy ...
Atomic Structure Notes
... The mass of an atom is mostly from the __protons___ and ____neutrons________. Find O on the periodic table. It’s mass is _16.00___ amu. It has _8_ protons. It must have _8_ neutrons. Electrically neutral atoms (as opposed to ions) have one electron for every proton. Fill in this chart for these neut ...
... The mass of an atom is mostly from the __protons___ and ____neutrons________. Find O on the periodic table. It’s mass is _16.00___ amu. It has _8_ protons. It must have _8_ neutrons. Electrically neutral atoms (as opposed to ions) have one electron for every proton. Fill in this chart for these neut ...
2nd Nine Weeks Benchmark Study Guide
... Scientists: Democritus coined the term atomos: uncuttable. He said all things are made up of atoms. Dalton wrote the atomic theory and said that atoms of one element are the same but different than atoms of another element. Thomson used cathode rays to discover the electron and created the Plum Pudd ...
... Scientists: Democritus coined the term atomos: uncuttable. He said all things are made up of atoms. Dalton wrote the atomic theory and said that atoms of one element are the same but different than atoms of another element. Thomson used cathode rays to discover the electron and created the Plum Pudd ...
Chapter 4 Atoms and Elements
... electrostatic repulsive force • neutrons increase the attractive nuclear force without increasing the repulsive electrostatic force • neutrons increase the stability of the nucleus and the atom as long as there are only a few more neutrons than protons ...
... electrostatic repulsive force • neutrons increase the attractive nuclear force without increasing the repulsive electrostatic force • neutrons increase the stability of the nucleus and the atom as long as there are only a few more neutrons than protons ...
3. atomic structure and periodic table
... less radioactive over time. Measure the abundance of 14C in the material to be tested. By use of the half-life of 14C work out how old the object is by working out how much it has decayed. ...
... less radioactive over time. Measure the abundance of 14C in the material to be tested. By use of the half-life of 14C work out how old the object is by working out how much it has decayed. ...
Chapter 10: Multi-‐Electron Atoms – Optical Excitations
... level of each electron in the outer shell is determined by two quantum numbers n and . Since there are ( 2 + 1) values of m and 2 values of ms, there are 2 ( 2 + 1) combinations that have the same energy. However, some of the degeneracy is removed by considering the effect of the following inte ...
... level of each electron in the outer shell is determined by two quantum numbers n and . Since there are ( 2 + 1) values of m and 2 values of ms, there are 2 ( 2 + 1) combinations that have the same energy. However, some of the degeneracy is removed by considering the effect of the following inte ...
Atomic Structure & the Periodic Table CHAPTERS 4 & 5
... Bohr in 1915; it is not completely correct, but it has many features that are ...
... Bohr in 1915; it is not completely correct, but it has many features that are ...
Effective Nuclear Charge
... photons. Electron produces negative virtual photon and proton produces positive virtual photon. So, they put out electricity fields around themselves. Now look at two charge particle with different sign (an electron and a proton). Proton emits positive virtual photons. Photon moves toward the electr ...
... photons. Electron produces negative virtual photon and proton produces positive virtual photon. So, they put out electricity fields around themselves. Now look at two charge particle with different sign (an electron and a proton). Proton emits positive virtual photons. Photon moves toward the electr ...
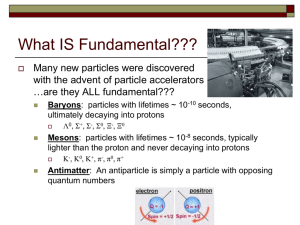
Lecture notes 6: Strong and weak interactions
... Every elementary particle has an antiparticle which can annihilate with its material counterpart. The antiparticle is the “opposite” of the particle in every way. For example the anti-proton, symbol −1 −1 p̄ is built of two anti up quarks and an anti down quark ūūd̄ so that it has baryon number −1 ...
... Every elementary particle has an antiparticle which can annihilate with its material counterpart. The antiparticle is the “opposite” of the particle in every way. For example the anti-proton, symbol −1 −1 p̄ is built of two anti up quarks and an anti down quark ūūd̄ so that it has baryon number −1 ...
Electricity T
... *Electrons are only transferred from one object to another. They are not ___created____ or destroyed. *There are ____3___ methods to transfer charge. 1. __friction_____ is the transfer of electrons from one object to another by ...
... *Electrons are only transferred from one object to another. They are not ___created____ or destroyed. *There are ____3___ methods to transfer charge. 1. __friction_____ is the transfer of electrons from one object to another by ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.