The Spectrum of Helium and Calcium
... Figure 1. Some energy levels of He, with an allowed and a forbidden transition to the ground state. 3c. Fine splitting. The 3 P levels in Fig. 1 are actually each split into three levels because S = 1 can add to L = 1 to give total angular momentum J = 0, 1, 2. Similarly, the 3 D levels are split in ...
... Figure 1. Some energy levels of He, with an allowed and a forbidden transition to the ground state. 3c. Fine splitting. The 3 P levels in Fig. 1 are actually each split into three levels because S = 1 can add to L = 1 to give total angular momentum J = 0, 1, 2. Similarly, the 3 D levels are split in ...
Chapter 5 Electrons in Atoms
... The better we know one, the less we know the other. Measuring changes the properties. True in quantum mechanics, but not classical mechanics ...
... The better we know one, the less we know the other. Measuring changes the properties. True in quantum mechanics, but not classical mechanics ...
Lecture 18
... By accelerating cathode rays through a known potential and then measuring the radius of their curved path in a known magnetic field, the charge to mass ratio could be measured: ...
... By accelerating cathode rays through a known potential and then measuring the radius of their curved path in a known magnetic field, the charge to mass ratio could be measured: ...
Do Now - White Plains Public Schools
... protons, electrons and neutrons. They are very different from each other in many ways. One way they are different is their "charge." Protons have a positive (+) charge. Electrons have a negative (-) charge. Neutrons have no charge. Usually, atoms have the same number of electrons and protons. Then t ...
... protons, electrons and neutrons. They are very different from each other in many ways. One way they are different is their "charge." Protons have a positive (+) charge. Electrons have a negative (-) charge. Neutrons have no charge. Usually, atoms have the same number of electrons and protons. Then t ...
Unit 4 Notes
... - Describe the relationship between wavelength and frequency of light - Identify the source of atomic emission spectra - Explain how the frequencies of light are related to changes in electron energies - Distinguish between quantum mechanics and classical physics - Know these terms: amplitude, wavel ...
... - Describe the relationship between wavelength and frequency of light - Identify the source of atomic emission spectra - Explain how the frequencies of light are related to changes in electron energies - Distinguish between quantum mechanics and classical physics - Know these terms: amplitude, wavel ...
atu_p_galla - Arkansas Space Grant Consortium
... were called shock waves. The phenomenon involving no mass motion was concluded to be from electron fluid action alone. Therefore they were referred to as EFD equations. Charles Wheatstone – In 1835 first speculated that the pulses observed from the discharge tube subjected to high potential differ ...
... were called shock waves. The phenomenon involving no mass motion was concluded to be from electron fluid action alone. Therefore they were referred to as EFD equations. Charles Wheatstone – In 1835 first speculated that the pulses observed from the discharge tube subjected to high potential differ ...
vg wbof
... As a result, l/L = (%)7T(4me 2/fi 2 k?. For electrons with energy E ~ 10 keV, the ratio l/L""' 0.03 and decreases with further increase of energy. Attention must be called to the fact that the beam electron spin precesses around the direction of the effective field J in the medium. Therefore, if the ...
... As a result, l/L = (%)7T(4me 2/fi 2 k?. For electrons with energy E ~ 10 keV, the ratio l/L""' 0.03 and decreases with further increase of energy. Attention must be called to the fact that the beam electron spin precesses around the direction of the effective field J in the medium. Therefore, if the ...
(TEQ) Model of the Electron - Superluminal quantum models of the
... Session T14: New Directions in Particle Theory May 4, 2009 www.superluminalquantum.org ...
... Session T14: New Directions in Particle Theory May 4, 2009 www.superluminalquantum.org ...
J.j. Thomson Atomic Theory — www.tutorvista.com — Readability
... Introduction to J.J.Thomson atomic theory:Thomson discovered the electron in the year 1897. His work put forward a new theory, that atom was made up of small particles.Thus he discovered the electrons. He proved his theory using the cathode ray tube. Scientists had already done many experiments to f ...
... Introduction to J.J.Thomson atomic theory:Thomson discovered the electron in the year 1897. His work put forward a new theory, that atom was made up of small particles.Thus he discovered the electrons. He proved his theory using the cathode ray tube. Scientists had already done many experiments to f ...
Chapter 5: Electrons in Atoms
... sublevels and orbitals of electrons in an atom. 2. Determine how to write electron configuration and orbital notation for atoms and ions. ...
... sublevels and orbitals of electrons in an atom. 2. Determine how to write electron configuration and orbital notation for atoms and ions. ...
Physics 212 Spring 2009 Exam 2 Version C
... Remember that by definition, the charge per unit time that passes through a given cross-sectional area is the current. Thus, the amount of chrage produced by the battery in one second is q = - I * (1 sec) = - 41 C. The charge of a single electron is e = -1.602 x 10-19 C. Thus, we have the number of ...
... Remember that by definition, the charge per unit time that passes through a given cross-sectional area is the current. Thus, the amount of chrage produced by the battery in one second is q = - I * (1 sec) = - 41 C. The charge of a single electron is e = -1.602 x 10-19 C. Thus, we have the number of ...
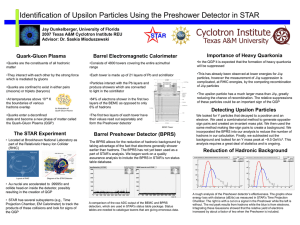
Dr. Saskia Mioduszewski Quark-Gluon Plasma
... Integrating these Gaussians showed that the relative yield of electrons increased by about a factor of two when the Preshower is included. ...
... Integrating these Gaussians showed that the relative yield of electrons increased by about a factor of two when the Preshower is included. ...
ATOMIC STRUCTURE
... These are the only particles that have been found in a stable nucleus. However, many other particles, such as positrons and mesons, have been observed during experiments involving nuclear disintegration. ...
... These are the only particles that have been found in a stable nucleus. However, many other particles, such as positrons and mesons, have been observed during experiments involving nuclear disintegration. ...
Water Molecule Conductivity
... Device to control 'color' of electrons in graphene provides path to future electronics A device made of bilayer graphene, an atomically thin hexagonal arrangement of carbon atoms, provides experimental proof of the ability to control the momentum of electrons and offers a path to electronics that c ...
... Device to control 'color' of electrons in graphene provides path to future electronics A device made of bilayer graphene, an atomically thin hexagonal arrangement of carbon atoms, provides experimental proof of the ability to control the momentum of electrons and offers a path to electronics that c ...
Electronic structure of correlated electron systems
... to exactly one electron per atom but the wave function would be a single Slater determinant of one electron Bloch waves and not a single Slater determinant of atomic site localized s orbitals with one electron at each site. In the DFT case there would be two electrons with opposite spin in each k st ...
... to exactly one electron per atom but the wave function would be a single Slater determinant of one electron Bloch waves and not a single Slater determinant of atomic site localized s orbitals with one electron at each site. In the DFT case there would be two electrons with opposite spin in each k st ...
1 slide per page() - Wayne State University Physics and Astronomy
... An absorption spectrum can be obtained by passing a continuous radiation spectrum through a vapor of the gas The absorption spectrum consists of a series of dark lines superimposed on the otherwise continuous spectrum ...
... An absorption spectrum can be obtained by passing a continuous radiation spectrum through a vapor of the gas The absorption spectrum consists of a series of dark lines superimposed on the otherwise continuous spectrum ...
Chapter 32 and 33 Review
... released. As they move, the force on each particle increases. Therefore, the particles have a. opposite signs. b. the same sign. c. charges that cannot be determined ...
... released. As they move, the force on each particle increases. Therefore, the particles have a. opposite signs. b. the same sign. c. charges that cannot be determined ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.