phys1144ch6p1
... If there are no nonconservative forces, the sum of the changes in the kinetic energy and in the potential energy is zero – the kinetic and potential energy changes are equal but opposite in sign. ...
... If there are no nonconservative forces, the sum of the changes in the kinetic energy and in the potential energy is zero – the kinetic and potential energy changes are equal but opposite in sign. ...
Diapositive 1 - indico in2p3
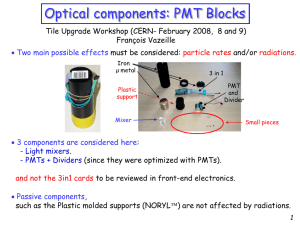
... Should be measured on some Light mixers after some LHC years. - Light mixers taken in the worst positions. - Needs access to the corresponding Drawers. 2. UV- dopant amount before starting LHC? as above. 3. SLHC effects: increase of above effects - Transparency decreased Light yield decrease exp ...
... Should be measured on some Light mixers after some LHC years. - Light mixers taken in the worst positions. - Needs access to the corresponding Drawers. 2. UV- dopant amount before starting LHC? as above. 3. SLHC effects: increase of above effects - Transparency decreased Light yield decrease exp ...
Ionic Bonding - cloudfront.net
... nitrogen and oxygen __________ C. phosphorus and chlorine __________ D. calcium and sulfur __________ E. chlorine and bromine ...
... nitrogen and oxygen __________ C. phosphorus and chlorine __________ D. calcium and sulfur __________ E. chlorine and bromine ...
ppt
... distributions in molecules but cannot explain the properties of some molecules. O O O 2: VB theory O: Is2 2s2 2p4 sp2 hybridized O, one sp2 from each forms s-bond and the other two are occupied with the lone pairs. The un-hybridized p on each forms the p-bond Indicates that in O2 molecule, all elect ...
... distributions in molecules but cannot explain the properties of some molecules. O O O 2: VB theory O: Is2 2s2 2p4 sp2 hybridized O, one sp2 from each forms s-bond and the other two are occupied with the lone pairs. The un-hybridized p on each forms the p-bond Indicates that in O2 molecule, all elect ...
Atomic Concepts
... 22. Lewis dot structure; a dot represents a valence electron (# dots = # valence electrons) 23. *** be able to calculate the atomic mass of an element, given masses and ratios of isotopes (% ÷ 100 * mass and add them all together) ...
... 22. Lewis dot structure; a dot represents a valence electron (# dots = # valence electrons) 23. *** be able to calculate the atomic mass of an element, given masses and ratios of isotopes (% ÷ 100 * mass and add them all together) ...
Determination of the Transport and Optical Properties of a Nonideal
... In conclusion, we emphasize that the dependences of the amplitude and phase of the reflected field of the short laser probe pulse on its delay time and heating pulse intensity that are obtained by means of the experimental procedure used in this work make it possible to acquire important information ...
... In conclusion, we emphasize that the dependences of the amplitude and phase of the reflected field of the short laser probe pulse on its delay time and heating pulse intensity that are obtained by means of the experimental procedure used in this work make it possible to acquire important information ...
Force - The Physics Doctor
... If an object is at equilibrium (i.e. moving at a constant speed or stationary) on a slope of a known angle, it’s weight will be acting as a vector (at an angle-just look, turn the image so the slope is horizontal!) This is the ONLY downward force and is being matched by the friction and reaction for ...
... If an object is at equilibrium (i.e. moving at a constant speed or stationary) on a slope of a known angle, it’s weight will be acting as a vector (at an angle-just look, turn the image so the slope is horizontal!) This is the ONLY downward force and is being matched by the friction and reaction for ...
Phys 2102 Spring 2002 - Louisiana State University
... How to charge an object • An object can be given some “excess” charge: giving electrons to it (we give it negative charge) or taking electrons away (we “give” it positive charge). • How do we do charge an object? Usually, moving charges from one surface to another by adhesion (helped by friction), ...
... How to charge an object • An object can be given some “excess” charge: giving electrons to it (we give it negative charge) or taking electrons away (we “give” it positive charge). • How do we do charge an object? Usually, moving charges from one surface to another by adhesion (helped by friction), ...