Chapter 29 Reflection and Refraction
... speed of light as it passes from one medium to another, or through varying temperatures and densities of the same medium – which changes the directions of light rays • Formula for the Index of Refraction (n) = (speed of light in vacuum)/(speed of light in material) ...
... speed of light as it passes from one medium to another, or through varying temperatures and densities of the same medium – which changes the directions of light rays • Formula for the Index of Refraction (n) = (speed of light in vacuum)/(speed of light in material) ...
UHM-A-634-2015-2-eye
... *There is a yellow filter between the first and second emulsion layer to prevent blue light from getting through to the lower layers because all silver halides are sensitive to blue light. *The film base is an orange color to reduce the contrast of the negative and to correct for sensitivities in th ...
... *There is a yellow filter between the first and second emulsion layer to prevent blue light from getting through to the lower layers because all silver halides are sensitive to blue light. *The film base is an orange color to reduce the contrast of the negative and to correct for sensitivities in th ...
eye-and-photography-2013
... *There is a yellow filter between the first and second emulsion layer to prevent blue light from getting through to the lower layers because all silver halides are sensitive to blue light. *The film base is an orange color to reduce the contrast of the negative and to correct for sensitivities in th ...
... *There is a yellow filter between the first and second emulsion layer to prevent blue light from getting through to the lower layers because all silver halides are sensitive to blue light. *The film base is an orange color to reduce the contrast of the negative and to correct for sensitivities in th ...
Radioactivity
... Heisenberg uncertainty : energy conservation can be violated as long as the violation does not last too long: ...
... Heisenberg uncertainty : energy conservation can be violated as long as the violation does not last too long: ...
19/Light and Sound in the Sea
... are sometimes located on the undersides of fish, which may serve some camouflage purpose. Many species of viperfish and anglerfish that live in deeper waters have bioluminescent lures that glow in the dark, an adaptation for attracting prey. Organisms may also use bioluminescence to see in the dark, ...
... are sometimes located on the undersides of fish, which may serve some camouflage purpose. Many species of viperfish and anglerfish that live in deeper waters have bioluminescent lures that glow in the dark, an adaptation for attracting prey. Organisms may also use bioluminescence to see in the dark, ...
Refraction - cashmerephysics
... As the sun begins to set, the light must travel farther through the atmosphere before it gets to you. More of the light is reflected and scattered. As less reaches you directly, the sun appears less bright. The colour of the sun itself appears to change, first to orange and then to red. This is beca ...
... As the sun begins to set, the light must travel farther through the atmosphere before it gets to you. More of the light is reflected and scattered. As less reaches you directly, the sun appears less bright. The colour of the sun itself appears to change, first to orange and then to red. This is beca ...
High intensity lasers - Institute of Physics
... (where water is transparent to Xrays but carbon is not), and timeresolved chemistry experiments. The interaction of high-intensity laser beams with other forms of matter, as well as gases, has also revealed some remarkable and useful phenomena. Reactions with solids are energetic enough to ionise at ...
... (where water is transparent to Xrays but carbon is not), and timeresolved chemistry experiments. The interaction of high-intensity laser beams with other forms of matter, as well as gases, has also revealed some remarkable and useful phenomena. Reactions with solids are energetic enough to ionise at ...
Physics 101 – HW#10 Solutions – Koskelo
... wavelengths crunch up and get reduced, by the formula λn = λ0 / n where λn and λ0 are the wavelengths of the light in the material and in the vacuum respectively. Plugging in ‘n’ and λ0 from part b: λn =2.9·10-7 m e.) What is its frequency? Mathematically: Using the equation v = f λ using the new v ...
... wavelengths crunch up and get reduced, by the formula λn = λ0 / n where λn and λ0 are the wavelengths of the light in the material and in the vacuum respectively. Plugging in ‘n’ and λ0 from part b: λn =2.9·10-7 m e.) What is its frequency? Mathematically: Using the equation v = f λ using the new v ...
PHYS-2020: General Physics II Course Lecture Notes Section XI
... dispersion. As we have seen, this results from the wavelength dependence of the index of refraction. 2. The dispersion of light through an air-glass interface is the principle behind a prism. ...
... dispersion. As we have seen, this results from the wavelength dependence of the index of refraction. 2. The dispersion of light through an air-glass interface is the principle behind a prism. ...
1. The electric pencil sharpener in Mrs. Brown`s classroom gets very
... from point A to point B and from point C to point D from point B to point D only from point B to point C only from point A to point B only ...
... from point A to point B and from point C to point D from point B to point D only from point B to point C only from point A to point B only ...
1601.07738v1
... space is only one out of many possible alternatives. In particular, another convention about clock synchronization can be chosen, which maintains the concept of absolute simultaneity between inertial frames. Particle tracking in the laboratory frame treats time as an independent variable. This fact ...
... space is only one out of many possible alternatives. In particular, another convention about clock synchronization can be chosen, which maintains the concept of absolute simultaneity between inertial frames. Particle tracking in the laboratory frame treats time as an independent variable. This fact ...
Electric Potential Energy
... Find the change in electric potential energy, U, as a charge of (a) 2.20 x 10-6 C or (b) -1.10 x 10-6 C moves from a point A to a point B, given that the change in electric potential between these points is V = VB – VA = 24.0 V. ...
... Find the change in electric potential energy, U, as a charge of (a) 2.20 x 10-6 C or (b) -1.10 x 10-6 C moves from a point A to a point B, given that the change in electric potential between these points is V = VB – VA = 24.0 V. ...
Slide 1
... •Relation between Electric Potential and Electric Field •Equipotential Lines •The Electron Volt, a Unit of Energy •Electric Potential Due to Point Charges •Potential Due to Electric Dipole; Dipole ...
... •Relation between Electric Potential and Electric Field •Equipotential Lines •The Electron Volt, a Unit of Energy •Electric Potential Due to Point Charges •Potential Due to Electric Dipole; Dipole ...
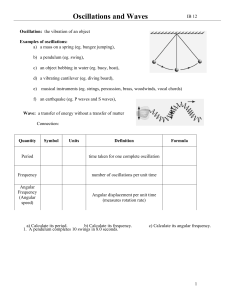
pkt 9 SHM and waves
... Natural Frequency of Vibration: when a system is displaced from equilibrium and allowed to oscillate freely, it will do so at its natural frequency of vibration Forced Oscillations – a system may be forced to oscillate at any given frequency by an outside force that is applied to it (called a drivin ...
... Natural Frequency of Vibration: when a system is displaced from equilibrium and allowed to oscillate freely, it will do so at its natural frequency of vibration Forced Oscillations – a system may be forced to oscillate at any given frequency by an outside force that is applied to it (called a drivin ...
Spectroscopy?
... The degree to which a dipole,P, is induced by the electric field, E, is described by the molecular polarizability, P= E is related to how readily the electrons will move under the influence of an electric field with respect to the nuclei of the molecule. If the molecule undergoes some internal ...
... The degree to which a dipole,P, is induced by the electric field, E, is described by the molecular polarizability, P= E is related to how readily the electrons will move under the influence of an electric field with respect to the nuclei of the molecule. If the molecule undergoes some internal ...
On Maxwell`s displacement current for energy and sensors: the
... We start from the very basic model of the TENG for illustrating its theory. Starting from a four layer TENG in contact-separation mode, with two dielectrics with permittivity of e1 and e2 and thicknesses d1 and d2, respectively (Fig. 4b-i). Once the two dielectrics are driven to be in physical conta ...
... We start from the very basic model of the TENG for illustrating its theory. Starting from a four layer TENG in contact-separation mode, with two dielectrics with permittivity of e1 and e2 and thicknesses d1 and d2, respectively (Fig. 4b-i). Once the two dielectrics are driven to be in physical conta ...