Water Soluble Vitamin
... • This class contain 8 B vitamins each work as coenzyme. So these vitamins must be present in every cell continuously for the cells function properly. Coenzyme: a small molecule that works with an enzyme to promote the enzymes activity. Many coenzymes have vitamin B as part of their structure. (CO=w ...
... • This class contain 8 B vitamins each work as coenzyme. So these vitamins must be present in every cell continuously for the cells function properly. Coenzyme: a small molecule that works with an enzyme to promote the enzymes activity. Many coenzymes have vitamin B as part of their structure. (CO=w ...
AP Biology Chapter 9 Cellular Respiration Guided Notes
... fermentation or anaerobic respiration and cannot survive in the presence of O2 • Yeast and many bacteria are ___________ __________, meaning that they can survive using either fermentation or cellular respiration • In a facultative anaerobe, __________ is a fork in the metabolic road that leads to t ...
... fermentation or anaerobic respiration and cannot survive in the presence of O2 • Yeast and many bacteria are ___________ __________, meaning that they can survive using either fermentation or cellular respiration • In a facultative anaerobe, __________ is a fork in the metabolic road that leads to t ...
09_Lecture_Presentation
... glucose → NADH → electron transport chain → proton-motive force → ATP About 34% of the energy in a glucose molecule is transferred to ATP during cellular respiration, making about 32 ATP There are several reasons why the number of ATP is not known exactly © 2014 Pearson Education, Inc. ...
... glucose → NADH → electron transport chain → proton-motive force → ATP About 34% of the energy in a glucose molecule is transferred to ATP during cellular respiration, making about 32 ATP There are several reasons why the number of ATP is not known exactly © 2014 Pearson Education, Inc. ...
A Supramolecular Peptide Synthesizer
... Once the macrocycle has converted the last amino acid, it is released from the axle and the target peptide can be detached from the macrocycle by hydrolysis. Remarkably, no starting material, deletions, or unexpected sequences were observed by HPLC-MS analysis and tandem MS. Several important molecu ...
... Once the macrocycle has converted the last amino acid, it is released from the axle and the target peptide can be detached from the macrocycle by hydrolysis. Remarkably, no starting material, deletions, or unexpected sequences were observed by HPLC-MS analysis and tandem MS. Several important molecu ...
Cellular Respiration - Esperanza High School
... requiring process that uses energy extracted from macromolecules (glucose) to produce energy (ATP) and water (H2O). C6H12O6 + 6O2 6CO2 + 6H2O + energy glucose ...
... requiring process that uses energy extracted from macromolecules (glucose) to produce energy (ATP) and water (H2O). C6H12O6 + 6O2 6CO2 + 6H2O + energy glucose ...
Dietary and Urinary Metabonomic Factors Possibly Accounting for
... Abstract—Black compared with non-Hispanic white Americans have higher systolic and diastolic blood pressure and rates of prehypertension/hypertension. Reasons for these adverse findings remain obscure. Analyses here focused on relations of foods/nutrients/urinary metabolites and higher black blood p ...
... Abstract—Black compared with non-Hispanic white Americans have higher systolic and diastolic blood pressure and rates of prehypertension/hypertension. Reasons for these adverse findings remain obscure. Analyses here focused on relations of foods/nutrients/urinary metabolites and higher black blood p ...
LECTURE 18 - Budostuff
... Describe how a H+ pumping mechanism is coupled to a proton-driven ATP synthase. State how many ATP molecules are produced per glucose molecule in the glycolytic pathway and in the whole respiratory pathway. Describe where in the respiratory pathway CO2 is released, and where O2 is consumed. ...
... Describe how a H+ pumping mechanism is coupled to a proton-driven ATP synthase. State how many ATP molecules are produced per glucose molecule in the glycolytic pathway and in the whole respiratory pathway. Describe where in the respiratory pathway CO2 is released, and where O2 is consumed. ...
m5zn_a9c640ccbe96115
... c) Myoglubin . d) Nothing . 51) Alpha helix differ from Beta sheet by……………………… a) H-bond exbend parallel to backbone . b) H-bond exbend vertical to backbone . c) Have only one polypeptide chain . d) A & C . 52) In alpha helix the H-bond performed between the carbonyl oxygen and.. a) Hydrogen of side ...
... c) Myoglubin . d) Nothing . 51) Alpha helix differ from Beta sheet by……………………… a) H-bond exbend parallel to backbone . b) H-bond exbend vertical to backbone . c) Have only one polypeptide chain . d) A & C . 52) In alpha helix the H-bond performed between the carbonyl oxygen and.. a) Hydrogen of side ...
Amino acid transport in Penicillium chrysogenum in relation to
... these compounds, suggests that the origin of these genes stems from prokaryotic organisms. In bacteria, the production of β-lactams may have evolved as a means of improving their ability to compete with other prokaryotes. ...
... these compounds, suggests that the origin of these genes stems from prokaryotic organisms. In bacteria, the production of β-lactams may have evolved as a means of improving their ability to compete with other prokaryotes. ...
... unit of collagen is tropocollagen, a rod-shaped protein consisting of three polypeptides unit (called α-chains) intertwined to form a triple-helical structure [1]. Each polypeptide chain forms a left-handed helix and consists of repeating triplets, (Gly-X-Y)n, where X and Y are, with a high possibil ...
vitamine
... intestines only in the presence of bile salts and other lipids through interaction with chylomicrons. Therefore, fat malabsorptive diseases can result in vitamin K deficiency. • Present in green leafy vegetables like lettuce, parsley, spinach and various greens (beet and mustard). Broccoli and certa ...
... intestines only in the presence of bile salts and other lipids through interaction with chylomicrons. Therefore, fat malabsorptive diseases can result in vitamin K deficiency. • Present in green leafy vegetables like lettuce, parsley, spinach and various greens (beet and mustard). Broccoli and certa ...
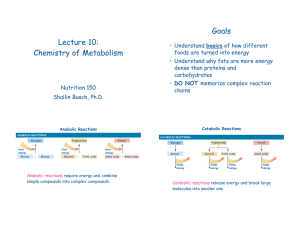
Lecture 10
... Anabolic reactions require energy and combine simple compounds into complex compounds. ...
... Anabolic reactions require energy and combine simple compounds into complex compounds. ...
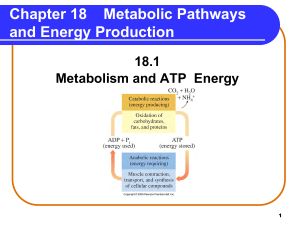
Metabolic Pathways and Energy Production
... • begin digestion in the mouth, where salivary amylase breaks down polysaccharides to smaller polysaccharides (dextrins), maltose, and some glucose. • continue digestion in the small intestine, where pancreatic amylase hydrolyzes dextrins to maltose and glucose. • maltose, lactose, and sucrose are h ...
... • begin digestion in the mouth, where salivary amylase breaks down polysaccharides to smaller polysaccharides (dextrins), maltose, and some glucose. • continue digestion in the small intestine, where pancreatic amylase hydrolyzes dextrins to maltose and glucose. • maltose, lactose, and sucrose are h ...
electron transport chain
... with no release of CO2 • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt • Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce ...
... with no release of CO2 • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt • Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce ...
Malo-ethanolic fermentation in Saccharomyces and
... of quality wines that require a judicious balance between the sugar, acid and flavour/aroma components. However, strains of Saccharomyces routinely used for wine fermentation in general do not degrade L-malic acid effectively during alcoholic fermentation. Furthermore, the degree of L-malic acid degra ...
... of quality wines that require a judicious balance between the sugar, acid and flavour/aroma components. However, strains of Saccharomyces routinely used for wine fermentation in general do not degrade L-malic acid effectively during alcoholic fermentation. Furthermore, the degree of L-malic acid degra ...
VITAMINS
... acid enhances iron absorption by keeping it in the ferrous form. This is due to reducing property of Vitamin C. it help in the formation of ferritin (storage form of iron) and metaboilzation of iron from ferritin. Vitamin C is useful in the reconversion of methemoglobin to hemoglogin. The degradatio ...
... acid enhances iron absorption by keeping it in the ferrous form. This is due to reducing property of Vitamin C. it help in the formation of ferritin (storage form of iron) and metaboilzation of iron from ferritin. Vitamin C is useful in the reconversion of methemoglobin to hemoglogin. The degradatio ...
Transport of Aromatic Amino Acids by Brevibacterium linens
... linens in the food industry and its considerable economic value, it has not been studied to a great extent. The genus Brevibacterium remains classed "genera incertae sedis" in Bergey's Manual of Determinative Bacteriology (8). In addition to its intense proteolytic action (13), this microorganism pa ...
... linens in the food industry and its considerable economic value, it has not been studied to a great extent. The genus Brevibacterium remains classed "genera incertae sedis" in Bergey's Manual of Determinative Bacteriology (8). In addition to its intense proteolytic action (13), this microorganism pa ...
Factors affecting human decomposition
... The body begins to dry out (mummification) and has a cheesy smell, caused by butyric acid. Adipocere formation (or saponification) may occur producing a yellow/white greasy wax-like substance. Adipocere develops as a result of fat hydrolysis releasing fatty acids. All the remaining flesh is removed ...
... The body begins to dry out (mummification) and has a cheesy smell, caused by butyric acid. Adipocere formation (or saponification) may occur producing a yellow/white greasy wax-like substance. Adipocere develops as a result of fat hydrolysis releasing fatty acids. All the remaining flesh is removed ...
this lecture as PDF here
... Lipids are anhydrous due to non-polar nature and represent more energy than carbohydrates which are heavily hydrated due to polar nature. The presence of lipids in diet contributes considerably to palatability. Lipids contribute palatability in two ways. They induce olfactory responses, namely ...
... Lipids are anhydrous due to non-polar nature and represent more energy than carbohydrates which are heavily hydrated due to polar nature. The presence of lipids in diet contributes considerably to palatability. Lipids contribute palatability in two ways. They induce olfactory responses, namely ...
COX-1 And COX-2 Enzymes Synthesize Prostaglandins and Are
... smooth endoplasmic reticulum. Cyclooxygenase inhibitors bind into the cyclooxygenase site of the enzyme, effectively inhibiting both that site and the peroxidase site. Because COX-1 and COX-2 are so similar, most NSAIDS inhibit both enzymes. Inhibiting COX-1, which protects your stomach, can create ...
... smooth endoplasmic reticulum. Cyclooxygenase inhibitors bind into the cyclooxygenase site of the enzyme, effectively inhibiting both that site and the peroxidase site. Because COX-1 and COX-2 are so similar, most NSAIDS inhibit both enzymes. Inhibiting COX-1, which protects your stomach, can create ...
Chapter 16 The Citric Acid Cycle
... dihydrolipoate, then NAD+ is reduced to NADH to reoxidize the FADH2 to complete the reaction. 37. Production of acetyl-CoA (activated acetate) Page: 605 Difficulty: 3 What is the function of FAD in the pyruvate dehydrogenase complex? How is it regenerated? Ans: FAD serves as the electron acceptor in ...
... dihydrolipoate, then NAD+ is reduced to NADH to reoxidize the FADH2 to complete the reaction. 37. Production of acetyl-CoA (activated acetate) Page: 605 Difficulty: 3 What is the function of FAD in the pyruvate dehydrogenase complex? How is it regenerated? Ans: FAD serves as the electron acceptor in ...
Review Ribosome-independent Peptide Synthesis in Nature and
... of proteins that are degraded via the pathway governed by N-end rule. These examples show the versatility and widespread importance of peptide bond in organisms. Interestingly, formations of these nonproteinous peptide bonds do not depend on the ribosome system. Specific enzymes are responsible for ...
... of proteins that are degraded via the pathway governed by N-end rule. These examples show the versatility and widespread importance of peptide bond in organisms. Interestingly, formations of these nonproteinous peptide bonds do not depend on the ribosome system. Specific enzymes are responsible for ...
Butyric acid
Butyric acid (from Greek βούτῡρον, meaning ""butter""), also known under the systematic name butanoic acid, abbreviated BTA, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. Salts and esters of butyric acid are known as butyrates or butanoates. Butyric acid is found in milk, especially goat, sheep and buffalo milk, butter, parmesan cheese, and as a product of anaerobic fermentation (including in the colon and as body odor). It has an unpleasant smell and acrid taste, with a sweetish aftertaste (similar to ether). It can be detected by mammals with good scent detection abilities (such as dogs) at 10 parts per billion, whereas humans can detect it in concentrations above 10 parts per million.Butyric acid is present in, and is the main distinctive smell of, human vomit.Butyric acid was first observed (in impure form) in 1814 by the French chemist Michel Eugène Chevreul. By 1818, he had purified it sufficiently to characterize it. The name of butyric acid comes from the Latin word for butter, butyrum (or buturum), the substance in which butyric acid was first found.