Conversion of amino acids to specialized products
... allergic and inflammatory reactions, gastric acid secretion, and possibly neurotransmission in the brain. ...
... allergic and inflammatory reactions, gastric acid secretion, and possibly neurotransmission in the brain. ...
The Complete Oxidation of Palmitate Yields 106 Molecules of ATP
... The pathway from propionyl CoA to succinyl CoA is especially interesting because it entails a rearrangement thatrequires vitamin B12 (also known as cobalamin). Propionyl CoA is carboxylated at the expense of the hydrolysis of an ATP to yield the d isomer of methylmalonyl CoA (Figure 22.11). This car ...
... The pathway from propionyl CoA to succinyl CoA is especially interesting because it entails a rearrangement thatrequires vitamin B12 (also known as cobalamin). Propionyl CoA is carboxylated at the expense of the hydrolysis of an ATP to yield the d isomer of methylmalonyl CoA (Figure 22.11). This car ...
Role of B vitamins in biological methylation – hdri
... affected by dietary levels of methyl-donor components, such as folic acid. This may be an important mechanism for environmentally-induced changes in gene expression. While methylation of cytosine is usually associated with the silencing of harmful genes, it may also activate genes in some instances. ...
... affected by dietary levels of methyl-donor components, such as folic acid. This may be an important mechanism for environmentally-induced changes in gene expression. While methylation of cytosine is usually associated with the silencing of harmful genes, it may also activate genes in some instances. ...
R-C-SCoA (acyl CoA) O
... organic acid (e.g. acetate) is an unactivated, low-energy, resonance-stabilized anion that is not easily attacked by a nucleophile (e.g. CoAS-); it is extremely difficult to remove one of the two carboxylate oxygen atoms in a displacement reaction. or this to occur the carboxylate group must be acti ...
... organic acid (e.g. acetate) is an unactivated, low-energy, resonance-stabilized anion that is not easily attacked by a nucleophile (e.g. CoAS-); it is extremely difficult to remove one of the two carboxylate oxygen atoms in a displacement reaction. or this to occur the carboxylate group must be acti ...
Brief Review Conjugated Linoleic Acid (CLA)
... Conjugated linoleic acid (CLA) is a term used for a large group of positional and geometric isomers of linoleic acid. Linoleic acid is an 18 carbon unsaturated, essential, dietary fatty acid (Steinhart 1996). Dietary sources for linoleic acid include the oils of vegetables, seeds, and nuts (Kelly et ...
... Conjugated linoleic acid (CLA) is a term used for a large group of positional and geometric isomers of linoleic acid. Linoleic acid is an 18 carbon unsaturated, essential, dietary fatty acid (Steinhart 1996). Dietary sources for linoleic acid include the oils of vegetables, seeds, and nuts (Kelly et ...
Metabolic fuels: regulating fluxes to select mix
... training and sympathetic stimulation may influence the crossover pattern of fuel selection, but whether these effects are significant has not been clearly demonstrated (Brooks and Mercier, 1994; Brooks, 1998; Bergman and Brooks, 1999). To determine the generality of such a fuel selection model, meas ...
... training and sympathetic stimulation may influence the crossover pattern of fuel selection, but whether these effects are significant has not been clearly demonstrated (Brooks and Mercier, 1994; Brooks, 1998; Bergman and Brooks, 1999). To determine the generality of such a fuel selection model, meas ...
Essential amino acid
... • Nitrogen oxide (NO) is a particularly interesting molecule: – Chemically, it is a free radical and therefore very reactive. – Biologically, it lowers blood pressure, kills invading bacteria, and enhances memory. – Nitric oxide is synthesized from oxygen and the amino acid arginine. – In blood vess ...
... • Nitrogen oxide (NO) is a particularly interesting molecule: – Chemically, it is a free radical and therefore very reactive. – Biologically, it lowers blood pressure, kills invading bacteria, and enhances memory. – Nitric oxide is synthesized from oxygen and the amino acid arginine. – In blood vess ...
Biologically Active Oxylipins from Enzymatic and Nonenzymatic
... and cymatherols [18,22,23,52]. Although extensive detail about oxylipin occurrence in microalgae is beyond this review, some important features of oxylipin metabolism in these unicellular organisms cannot be discarded. A characteristic difference from macroalgae is the complete absence of C18 PUFA-d ...
... and cymatherols [18,22,23,52]. Although extensive detail about oxylipin occurrence in microalgae is beyond this review, some important features of oxylipin metabolism in these unicellular organisms cannot be discarded. A characteristic difference from macroalgae is the complete absence of C18 PUFA-d ...
EFFECT OF COOKING AND ROASTING ON THE AMINO ACID
... is the raw sample. About 350 g of the dried groundnut pods were put into an iron pot and mixed with clean fine sand and stirred to prevent burning of the sample and to ensure uniform distribution of heat. The groundnut pods were roasted for about 30 min at 120-130°C using Gallenkamp thermostat hot p ...
... is the raw sample. About 350 g of the dried groundnut pods were put into an iron pot and mixed with clean fine sand and stirred to prevent burning of the sample and to ensure uniform distribution of heat. The groundnut pods were roasted for about 30 min at 120-130°C using Gallenkamp thermostat hot p ...
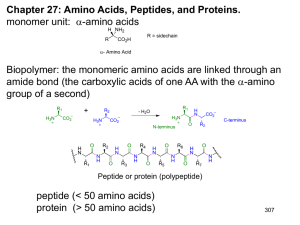
ppt - Vanderbilt University
... Hydrophobic effect. Proteins will fold so that hydrophobic amino acids are on the inside (shielded from water) and hydrophilic amino acids are on the outside (exposed to water). ...
... Hydrophobic effect. Proteins will fold so that hydrophobic amino acids are on the inside (shielded from water) and hydrophilic amino acids are on the outside (exposed to water). ...
The Citric Acid Cycle
... •The first of two oxidative decarboxylations in the cycle is catalyzed by isocitrate dehydrogenase. •Isocitrate is oxidized to a ketone, oxalosuccinate, an unstable •enzyme-bound intermediate that spontaneously b-decarboxylates to give the product, a-ketoglutarate. •The strategy here is to oxidize i ...
... •The first of two oxidative decarboxylations in the cycle is catalyzed by isocitrate dehydrogenase. •Isocitrate is oxidized to a ketone, oxalosuccinate, an unstable •enzyme-bound intermediate that spontaneously b-decarboxylates to give the product, a-ketoglutarate. •The strategy here is to oxidize i ...
Ch 6 LIPID METABOLISM - FORMATTED - NSDL
... The figure below demarcates the major sites of lipid metabolism within an animal cell like the hepatocyte: The reactions of lipid synthesis and catabolism occur simultaneously within the cell but are segregated in different areas so that futile cycles are prevented. Thus lipid synthesis takes place ...
... The figure below demarcates the major sites of lipid metabolism within an animal cell like the hepatocyte: The reactions of lipid synthesis and catabolism occur simultaneously within the cell but are segregated in different areas so that futile cycles are prevented. Thus lipid synthesis takes place ...
Ch20.1 Amino-acids-degradation and synthesis
... 3. Catabolism of the Carbon Skeletons of Amino Acids 2. Isoleucine: This amino acid is both ketogenic and glucogenic, because its metabolism yields acetyl CoA and propionyl CoA. The first three steps in the metabolism of isoleucine are virtually identical to the initial steps in the degradation o ...
... 3. Catabolism of the Carbon Skeletons of Amino Acids 2. Isoleucine: This amino acid is both ketogenic and glucogenic, because its metabolism yields acetyl CoA and propionyl CoA. The first three steps in the metabolism of isoleucine are virtually identical to the initial steps in the degradation o ...
cellular-respiration 1
... a. NADH gives up its electrons and becomes NAD+; the next carrier then gains electrons and is thereby reduced. b. At each sequential redox reaction, energy is released to form ATP molecules. c. Some of the protein carriers are cytochrome molecules, complex carbon rings with a heme (iron) group in t ...
... a. NADH gives up its electrons and becomes NAD+; the next carrier then gains electrons and is thereby reduced. b. At each sequential redox reaction, energy is released to form ATP molecules. c. Some of the protein carriers are cytochrome molecules, complex carbon rings with a heme (iron) group in t ...
2.2.56. amino acid analysis
... amino acid analysis of these molecules. The glassware used for hydrolysis must be very clean to avoid erroneous results. Glove powders and fingerprints on hydrolysis tubes may cause contamination. To clean glass hydrolysis tubes, boil tubes for 1 h in 1 M hydrochloric acid or soak tubes in concentra ...
... amino acid analysis of these molecules. The glassware used for hydrolysis must be very clean to avoid erroneous results. Glove powders and fingerprints on hydrolysis tubes may cause contamination. To clean glass hydrolysis tubes, boil tubes for 1 h in 1 M hydrochloric acid or soak tubes in concentra ...
Cellular Respiration I - hrsbstaff.ednet.ns.ca
... 8.1.1 State that oxidation involves the loss of electrons from an element, whereas reduction involves a gain of electrons; and that oxidation frequently involves gaining oxygen or losing hydrogen, whereas reduction frequently involves losing oxygen or gaining hydrogen. 8.1.2 Outline the process of g ...
... 8.1.1 State that oxidation involves the loss of electrons from an element, whereas reduction involves a gain of electrons; and that oxidation frequently involves gaining oxygen or losing hydrogen, whereas reduction frequently involves losing oxygen or gaining hydrogen. 8.1.2 Outline the process of g ...
Lesson 3.Carbohydrate Metabolism
... Gluconeogenesis is a pathway consisting of eleven enzyme-catalyzed reactions. The pathway can begin in the mitochondria or cytoplasm, depending on the substrate being used. Many of the reactions are reversible steps found in glycolysis. Gluconeogenesis begins in the mitochondria with the formation o ...
... Gluconeogenesis is a pathway consisting of eleven enzyme-catalyzed reactions. The pathway can begin in the mitochondria or cytoplasm, depending on the substrate being used. Many of the reactions are reversible steps found in glycolysis. Gluconeogenesis begins in the mitochondria with the formation o ...
Practical part
... 8. Objective of biochemical investigations: the whole organism and its organs, tissues, cells, homogenates, subcellular organelles, extracts and molecular biocomplexes. 9. Clinical and diagnostic significance of biochemical investigation. 10. Biological material used in biochemical investigations. 1 ...
... 8. Objective of biochemical investigations: the whole organism and its organs, tissues, cells, homogenates, subcellular organelles, extracts and molecular biocomplexes. 9. Clinical and diagnostic significance of biochemical investigation. 10. Biological material used in biochemical investigations. 1 ...
odour away
... odours caused by hydrogen sulphide. PNSB are also very effective at decomposing organic matter under anaerobic conditions without the production of odiferous compounds such as hydrogen sulphide and volatile organic compounds and in this way compete with microbes that cause putrefaction and its assoc ...
... odours caused by hydrogen sulphide. PNSB are also very effective at decomposing organic matter under anaerobic conditions without the production of odiferous compounds such as hydrogen sulphide and volatile organic compounds and in this way compete with microbes that cause putrefaction and its assoc ...
Chapter 8 Cellular Respiration 8.1 Cellular Respiration 1. Cellular
... 1. The electron transport chain is located in the cristae of mitochondria and consists of carriers that pass electrons successively from one to another. 2. NADH and FADH2 carry the electrons to the electron transport system. 3. Members of the Chain a. NADH gives up its electrons and becomes NAD+; th ...
... 1. The electron transport chain is located in the cristae of mitochondria and consists of carriers that pass electrons successively from one to another. 2. NADH and FADH2 carry the electrons to the electron transport system. 3. Members of the Chain a. NADH gives up its electrons and becomes NAD+; th ...
Azospirillum and related microorganisms.
... and leaves from different plants in rather high numbers. Interestingly, it could be demonstrated that the mild plant pathogenic Pseudomonas rubrislibalbicans is related to Herbaspirillum seropedicae on the genus level (Gillis et al. 1991, Hartmann et al., 1994). Molecular taxonomy is a powerful tool ...
... and leaves from different plants in rather high numbers. Interestingly, it could be demonstrated that the mild plant pathogenic Pseudomonas rubrislibalbicans is related to Herbaspirillum seropedicae on the genus level (Gillis et al. 1991, Hartmann et al., 1994). Molecular taxonomy is a powerful tool ...
Cellular Respiration
... • Before the citric acid cycle can begin, pyruvate must be converted to acetyl CoA – Prior to this, one C from pyruvate is removed as CO2 – CoA = coenzyme A (organic substance that helps enzymes function) ...
... • Before the citric acid cycle can begin, pyruvate must be converted to acetyl CoA – Prior to this, one C from pyruvate is removed as CO2 – CoA = coenzyme A (organic substance that helps enzymes function) ...
Chapter 8 Cellular Respiration Dr. Harold Kay Njemanze 8.1
... 1. The electron transport chain is located in the cristae of mitochondria and consists of carriers that pass electrons successively from one to another. 2. NADH and FADH2 carry the electrons to the electron transport system. 3. Members of the Chain a. NADH gives up its electrons and becomes NAD+; th ...
... 1. The electron transport chain is located in the cristae of mitochondria and consists of carriers that pass electrons successively from one to another. 2. NADH and FADH2 carry the electrons to the electron transport system. 3. Members of the Chain a. NADH gives up its electrons and becomes NAD+; th ...
Butyric acid
Butyric acid (from Greek βούτῡρον, meaning ""butter""), also known under the systematic name butanoic acid, abbreviated BTA, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. Salts and esters of butyric acid are known as butyrates or butanoates. Butyric acid is found in milk, especially goat, sheep and buffalo milk, butter, parmesan cheese, and as a product of anaerobic fermentation (including in the colon and as body odor). It has an unpleasant smell and acrid taste, with a sweetish aftertaste (similar to ether). It can be detected by mammals with good scent detection abilities (such as dogs) at 10 parts per billion, whereas humans can detect it in concentrations above 10 parts per million.Butyric acid is present in, and is the main distinctive smell of, human vomit.Butyric acid was first observed (in impure form) in 1814 by the French chemist Michel Eugène Chevreul. By 1818, he had purified it sufficiently to characterize it. The name of butyric acid comes from the Latin word for butter, butyrum (or buturum), the substance in which butyric acid was first found.