The Two Slit Experiment
... that there is a beam of electrons incident normally on a screen with the two slits, with all the electrons having the same energy E and momentum p. The screen is a fluorescent screen, so that the arrival of each electron is registered as a flash of light – the signature of the arrival of a particle ...
... that there is a beam of electrons incident normally on a screen with the two slits, with all the electrons having the same energy E and momentum p. The screen is a fluorescent screen, so that the arrival of each electron is registered as a flash of light – the signature of the arrival of a particle ...
Spin The evidence of intrinsic angular momentum or spin and its
... as is the case with orbital angular momentum, where the ratio q/2m is called Bohr magneton, µb and g is known as Lande-g factor or gyromagnetic factor. The g-factor is 1 for orbital angular momentum and hence corresponding magnetic moment is l µb (where l is integer orbital angular momentum quantum ...
... as is the case with orbital angular momentum, where the ratio q/2m is called Bohr magneton, µb and g is known as Lande-g factor or gyromagnetic factor. The g-factor is 1 for orbital angular momentum and hence corresponding magnetic moment is l µb (where l is integer orbital angular momentum quantum ...
Waveguides, Resonant Cavities, Optical Fibers and
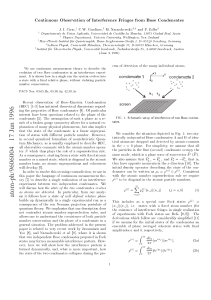
... potentials. The basis of this analogy is the fact that both wave equations for electromagnetic monoenergetic waves (i.e. with well-defined frequency), obtained directly from the Maxwell equations, and the time-independent Schrodinger equation are Helmholtz equations; when specific restrictions - like ...
... potentials. The basis of this analogy is the fact that both wave equations for electromagnetic monoenergetic waves (i.e. with well-defined frequency), obtained directly from the Maxwell equations, and the time-independent Schrodinger equation are Helmholtz equations; when specific restrictions - like ...
Quantum Mechanical Cross Sections
... kinematics which can contribute to the cross section. The cross section for scattering into DW is then obtained as an integral over all the allowed momenta for that solid angle. In the case of potential scattering we will assume only elastic scattering is allowed. This means that there is a change i ...
... kinematics which can contribute to the cross section. The cross section for scattering into DW is then obtained as an integral over all the allowed momenta for that solid angle. In the case of potential scattering we will assume only elastic scattering is allowed. This means that there is a change i ...
Glossary - Chemistry (Intro)
... Proton: A subatomic particle having a single positive electric mp = 1.67252⋅10-24 [g] charge. The mass of a proton is about 1840 times that of an electron. qp = +1.6022⋅10-19 [C] Aufbau Principle: As protons are added one by one to the nucleus to build up the elements, electrons similarly are added ...
... Proton: A subatomic particle having a single positive electric mp = 1.67252⋅10-24 [g] charge. The mass of a proton is about 1840 times that of an electron. qp = +1.6022⋅10-19 [C] Aufbau Principle: As protons are added one by one to the nucleus to build up the elements, electrons similarly are added ...
Word
... It is generally no longer consistent to talk of individual entities possessing a value for an observable: it is more as if the property concerned is latent in the system until such time as an observation brings it into being. As John Bell has put it, "beables" are replaced by "observables," and the ...
... It is generally no longer consistent to talk of individual entities possessing a value for an observable: it is more as if the property concerned is latent in the system until such time as an observation brings it into being. As John Bell has put it, "beables" are replaced by "observables," and the ...
C. Heitzinger, C. Ringhofer. S. Ahmed, D. Vasileska
... Abstract. Effective quantum potentials describe the physics of quantum-mechanical electron transport in semiconductors more than the classical Coulomb potential. An effective quantum potential was derived previously for the interaction of an electron with a barrier for use in particle-based Monte Ca ...
... Abstract. Effective quantum potentials describe the physics of quantum-mechanical electron transport in semiconductors more than the classical Coulomb potential. An effective quantum potential was derived previously for the interaction of an electron with a barrier for use in particle-based Monte Ca ...
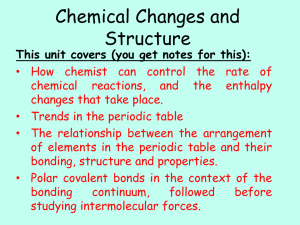
Chemistry I
... higher level (outer orbit). If photon energy too high ⇒ ionization Deactivation of excited electrons under emission of electromagnetic wave and return to lower level (inner orbit). Deactivation by emission of light ...
... higher level (outer orbit). If photon energy too high ⇒ ionization Deactivation of excited electrons under emission of electromagnetic wave and return to lower level (inner orbit). Deactivation by emission of light ...
mark scheme - A-Level Chemistry
... Multiply m/z by relative abundance for each isotope (1) Allow instead of m/z mass no, Ar or actual value from example Sum these values (1) Divide by the sum of the relative abundances (1) only award this mark if previous 2 given Max 2 if e.g. has only 2 isotopes ...
... Multiply m/z by relative abundance for each isotope (1) Allow instead of m/z mass no, Ar or actual value from example Sum these values (1) Divide by the sum of the relative abundances (1) only award this mark if previous 2 given Max 2 if e.g. has only 2 isotopes ...
Performance of Many–Body Perturbation Theory
... estimate the size of the neglected effects, but there is no such method (at least not yet), see section 2.2.2. For a small number of electrons the CI-approach can produce virtually exact results, provided of course that the basis set describes the physical space well enough. The size of the full CI ...
... estimate the size of the neglected effects, but there is no such method (at least not yet), see section 2.2.2. For a small number of electrons the CI-approach can produce virtually exact results, provided of course that the basis set describes the physical space well enough. The size of the full CI ...
Name - TeacherWeb
... The elements in Group 18 are known as the noble gases. They do not usually form compounds because they do not like to gain, lose, or share electrons. All of the noble gases exist in the Earth’s atmosphere, but only in small amounts. ...
... The elements in Group 18 are known as the noble gases. They do not usually form compounds because they do not like to gain, lose, or share electrons. All of the noble gases exist in the Earth’s atmosphere, but only in small amounts. ...
Electrochemistry
... Balancing Redox Equations Redox reactions are often quite complicated and difficult to balance. For this reason, you’ll learn a step-by-step method for balancing these types of reactions, when they occur in acidic or in basic solutions. The procedure is called the “Half-Reactions Method” of balanci ...
... Balancing Redox Equations Redox reactions are often quite complicated and difficult to balance. For this reason, you’ll learn a step-by-step method for balancing these types of reactions, when they occur in acidic or in basic solutions. The procedure is called the “Half-Reactions Method” of balanci ...
12 U Chem Review
... electrons travel in the atom in circular orbits with quantized energy – energy is restricted to only certain discrete quantities there is a maximum number of electrons allowed in each orbit electrons “jump” to a higher level when a photon (a quantum of light energy) is absorbed, resulting in absorpt ...
... electrons travel in the atom in circular orbits with quantized energy – energy is restricted to only certain discrete quantities there is a maximum number of electrons allowed in each orbit electrons “jump” to a higher level when a photon (a quantum of light energy) is absorbed, resulting in absorpt ...
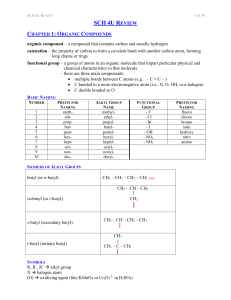
sch4ureview
... electrons travel in the atom in circular orbits with quantized energy – energy is restricted to only certain discrete quantities there is a maximum number of electrons allowed in each orbit electrons “jump” to a higher level when a photon (a quantum of light energy) is absorbed, resulting in absorpt ...
... electrons travel in the atom in circular orbits with quantized energy – energy is restricted to only certain discrete quantities there is a maximum number of electrons allowed in each orbit electrons “jump” to a higher level when a photon (a quantum of light energy) is absorbed, resulting in absorpt ...
AP Chemistry Name: Ch.1 – Matter and Measurement Date: Period:
... experimental data. Various scoops of jelly beans were weighed and the following masses determined. The number of jelly beans in each scoop was not known. Masses (in grams) of ten different scoops: ...
... experimental data. Various scoops of jelly beans were weighed and the following masses determined. The number of jelly beans in each scoop was not known. Masses (in grams) of ten different scoops: ...
atom-ph/9606004 PDF
... evolve apart from a decay of its amplitude. The distribution of atoms in the screen corresponding R^( ) is given by P ( ) / ja + bei( ) j = a2 + b2 + 2ab cos( ) (14) and agrees perfectly with the interference pattern that one would expect intuitively from a state of xed relative phase . An inter ...
... evolve apart from a decay of its amplitude. The distribution of atoms in the screen corresponding R^( ) is given by P ( ) / ja + bei( ) j = a2 + b2 + 2ab cos( ) (14) and agrees perfectly with the interference pattern that one would expect intuitively from a state of xed relative phase . An inter ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.