Quantum theory
... Electrons in the same orbital must coexist together How if they are repulsive Fourth quantum number is spin s Electrons in the same orbital spin in opposite directions • Sets up opposite magnetic fields, so e- become slightly attractive to each other • Up and down arrows E used to show spin directio ...
... Electrons in the same orbital must coexist together How if they are repulsive Fourth quantum number is spin s Electrons in the same orbital spin in opposite directions • Sets up opposite magnetic fields, so e- become slightly attractive to each other • Up and down arrows E used to show spin directio ...
energy - Edublogs
... • There is no such thing as a half of an electron or a fifth of a proton, so everything that has electrical charge must have some multiple of the charge of an electron or proton- 5 electrons, 8 protons, etc. That’s why electric charge is QUANTIZED. ...
... • There is no such thing as a half of an electron or a fifth of a proton, so everything that has electrical charge must have some multiple of the charge of an electron or proton- 5 electrons, 8 protons, etc. That’s why electric charge is QUANTIZED. ...
ChemFinalgeocities
... One isotope of carbon has 6 protons and 6 neutrons. The number of protons and neutrons of a second isotope of carbon would be _____. a. 7 and 6 c. 7 and 7 b. 6 and 7 d. 6 and 6 According to the law of conservation of matter, if 4.0 g of hydrogen react with chlorine to produce 146 g of hydrogen chlor ...
... One isotope of carbon has 6 protons and 6 neutrons. The number of protons and neutrons of a second isotope of carbon would be _____. a. 7 and 6 c. 7 and 7 b. 6 and 7 d. 6 and 6 According to the law of conservation of matter, if 4.0 g of hydrogen react with chlorine to produce 146 g of hydrogen chlor ...
Chem700 MO
... in a different spin orbital (or else the determinant is zero) • an electron has both space and spin coordinates • an electron can be alpha spin (, , spin up) or beta spin ...
... in a different spin orbital (or else the determinant is zero) • an electron has both space and spin coordinates • an electron can be alpha spin (, , spin up) or beta spin ...
Introduction of New Products
... The AccuTOF GCv 4G is a fully automated Gas Chromatograph Time-of-Flight Mass Spectrometer (GC-TOFMS). A data recording speed of 50 spectra/second enables high throughput analysis. A new high speed preamp and data acquisition system is capable of sampling TOFMS signal at 4 giga samples/second, twice ...
... The AccuTOF GCv 4G is a fully automated Gas Chromatograph Time-of-Flight Mass Spectrometer (GC-TOFMS). A data recording speed of 50 spectra/second enables high throughput analysis. A new high speed preamp and data acquisition system is capable of sampling TOFMS signal at 4 giga samples/second, twice ...
Quantum Mechanics: PHL555 Tutorial 2
... part of the Hamiltonian represents the interaction with the magnetic field. We have neglected the effects due to spin angular momentum of the electron . Treat H 1 as a perturbation and show s(l 0) states are not split , where as p(l 1) states are split into three states separated by the energy i ...
... part of the Hamiltonian represents the interaction with the magnetic field. We have neglected the effects due to spin angular momentum of the electron . Treat H 1 as a perturbation and show s(l 0) states are not split , where as p(l 1) states are split into three states separated by the energy i ...
Chemistry Study Guide
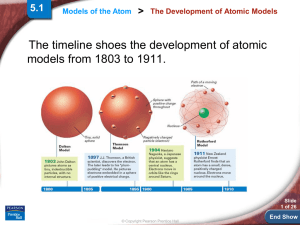
... Called Bohr models: We show the protons and neutrons in the nucleus and electrons in circular “clouds” outside the nucleus. Electron clouds can hold , 2, 8, then 18 electrons from the inside ring out. We fill the inside ring first. He ...
... Called Bohr models: We show the protons and neutrons in the nucleus and electrons in circular “clouds” outside the nucleus. Electron clouds can hold , 2, 8, then 18 electrons from the inside ring out. We fill the inside ring first. He ...
Covalent Bonding - Effingham County Schools
... •As independent particles, most atoms are at relatively high potential energy. •Nature, however, favors arrangements in which potential energy is minimized. •This means that most atoms are less stable existing by themselves than when they are combined. •By bonding with each other, atoms decrease in ...
... •As independent particles, most atoms are at relatively high potential energy. •Nature, however, favors arrangements in which potential energy is minimized. •This means that most atoms are less stable existing by themselves than when they are combined. •By bonding with each other, atoms decrease in ...
De Broglie Waves, Uncertainty, and Atoms
... Black side is hotter:gas molecules bounce off it with more momentum than on shiny side-this is a bigger effect than the photon momentum ...
... Black side is hotter:gas molecules bounce off it with more momentum than on shiny side-this is a bigger effect than the photon momentum ...
C1 Revision Fundamental ideas adapted CS
... non-metals g............... electrons to form n....................... ions Complete the labels on the diagrams to show formation of sodium chloride (only outer shell electrons are shown): ...
... non-metals g............... electrons to form n....................... ions Complete the labels on the diagrams to show formation of sodium chloride (only outer shell electrons are shown): ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... a simple solution, which assumes the wave function to depend only on the distance r and not on θ and φ. b) The wave function of 1s orbital of Li2+ is Ψ1s = (1/√π) (Z/a0)3/2 exp(-Zr/a0), where a0 is the most probable distance of the electron from the nucleus and Z is the atomic number. Show that the ...
... a simple solution, which assumes the wave function to depend only on the distance r and not on θ and φ. b) The wave function of 1s orbital of Li2+ is Ψ1s = (1/√π) (Z/a0)3/2 exp(-Zr/a0), where a0 is the most probable distance of the electron from the nucleus and Z is the atomic number. Show that the ...
Chem vocab quiz definitons
... Liquid is the state of matter that is described as having a definite volume but an indefinite shape. Gas is the state of matter that is described as having no definite shape, or volume. Solid is the state of matter that is described as having a definite shape and volume. Viscosity is a property of l ...
... Liquid is the state of matter that is described as having a definite volume but an indefinite shape. Gas is the state of matter that is described as having no definite shape, or volume. Solid is the state of matter that is described as having a definite shape and volume. Viscosity is a property of l ...
Elements and the Periodic Table
... Halogens form diatomic molecules (Cl2, F2) They are highly reactive, and toxic to many organisms. ...
... Halogens form diatomic molecules (Cl2, F2) They are highly reactive, and toxic to many organisms. ...
The Quantum Hypothesis slides
... Light can act as a wave or a particle The energy of light is directly proportional to its frequency ...
... Light can act as a wave or a particle The energy of light is directly proportional to its frequency ...
Electrons and Atoms
... • These are called the atomic emission spectrum • Unique to each element, like fingerprints! • Very useful for identifying elements ...
... • These are called the atomic emission spectrum • Unique to each element, like fingerprints! • Very useful for identifying elements ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.