Nonlinear THz response of a one-dimensional superlattice * Avik W. Ghosh
... Recently, there has been considerable theoretical and experimental progress in studying the dynamics of charges in a semiconductor superlattice in response to intense ultrafast external fields. Following the suggestions of Esaki and Tsu,1 fabrication of semiconductor superlattices with atomic level ...
... Recently, there has been considerable theoretical and experimental progress in studying the dynamics of charges in a semiconductor superlattice in response to intense ultrafast external fields. Following the suggestions of Esaki and Tsu,1 fabrication of semiconductor superlattices with atomic level ...
Hong-Ou-Mandel interference between triggered and heralded
... sources respectively, ts is the single atom excitation instant following a heralding event at tf , and Θ(t) is the Heaviside step function. For the single atom, we confirm τs = 26.18 ± 0.11 ns, corresponding to the natural linewidth of the transition. For the FWM source, τf = 13.61 ± 0.73 ns, where ...
... sources respectively, ts is the single atom excitation instant following a heralding event at tf , and Θ(t) is the Heaviside step function. For the single atom, we confirm τs = 26.18 ± 0.11 ns, corresponding to the natural linewidth of the transition. For the FWM source, τf = 13.61 ± 0.73 ns, where ...
Theoretical modeling of x-ray and vibrational spectroscopies applied to liquid
... A spectroscopic measurement is one of the most fundamental methods to probe matter. By measuring the interaction of the system of study with light or particles as a function of energy a lot of information can be gained about, for example, the positions of the atoms, the electronic structure, or how ...
... A spectroscopic measurement is one of the most fundamental methods to probe matter. By measuring the interaction of the system of study with light or particles as a function of energy a lot of information can be gained about, for example, the positions of the atoms, the electronic structure, or how ...
CHAPTER I
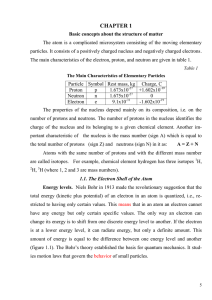
... Copper, in Group IB, will also have one electron assigned to the 4s orbital, plus 28 other electrons assigned to other orbitals. The configuration of Be 1s2 2s2.All elements of Group 2A have electron configurations [electrons of preceding rare gas + ns2], where n is the period in which the element ...
... Copper, in Group IB, will also have one electron assigned to the 4s orbital, plus 28 other electrons assigned to other orbitals. The configuration of Be 1s2 2s2.All elements of Group 2A have electron configurations [electrons of preceding rare gas + ns2], where n is the period in which the element ...
here.
... this vector has projection on the z-axis of ~m. So we know its angle with the z-axis. But that is as much as we can say. We cannot unambiguously specify its projections on x or y axes since L x and Ly do not have definite values in this state. To visualize the ‘angular momentum vector’ in this state ...
... this vector has projection on the z-axis of ~m. So we know its angle with the z-axis. But that is as much as we can say. We cannot unambiguously specify its projections on x or y axes since L x and Ly do not have definite values in this state. To visualize the ‘angular momentum vector’ in this state ...
Electron Attraction Mediated by Coulomb Repulsion
... attract each other not by a ‘phononic’ glue, but rather by their repulsion from other electrons. He predicted that if electrons, which are much lighter than ions, form the binding glue, pairing would be dramatically stronger than in conventional superconductors. To test this new form of attraction, ...
... attract each other not by a ‘phononic’ glue, but rather by their repulsion from other electrons. He predicted that if electrons, which are much lighter than ions, form the binding glue, pairing would be dramatically stronger than in conventional superconductors. To test this new form of attraction, ...
Get PDF - OSA Publishing
... In quantum optics, the system–reservoir model is treated with many different approaches. In the Schrödinger picture, this model has been treated in the master equation approach, where multiple-time averages are calculated via the quantum regression theorem [2]. Alternatively in the Schrödinger pictu ...
... In quantum optics, the system–reservoir model is treated with many different approaches. In the Schrödinger picture, this model has been treated in the master equation approach, where multiple-time averages are calculated via the quantum regression theorem [2]. Alternatively in the Schrödinger pictu ...
Local structure relaxation, quantum trap depression, and
... six considered different nanoclusters is demonstrated (see Table 1) in the calculations using Mulliken charge population analysis.57 The negative values in Table 1 represents charge gain, and the positive values charge loss. It can be seen that, for the considered structures, the electrons transfer ...
... six considered different nanoclusters is demonstrated (see Table 1) in the calculations using Mulliken charge population analysis.57 The negative values in Table 1 represents charge gain, and the positive values charge loss. It can be seen that, for the considered structures, the electrons transfer ...
CHE 110 Dr. Nicholas Bizier Office DS 337b email
... Lysine is an amino acid which has the following elemental composition: C, H, O, N. In one experiment, 2.175 g of lysine was combusted to produce 3.94 g of CO2 and 1.89 g H2O. In a separate experiment, 1.873 g of lysine was burned to produce 0.436 g of NH2. The molar mass of lysine is 150 g/mol. Dete ...
... Lysine is an amino acid which has the following elemental composition: C, H, O, N. In one experiment, 2.175 g of lysine was combusted to produce 3.94 g of CO2 and 1.89 g H2O. In a separate experiment, 1.873 g of lysine was burned to produce 0.436 g of NH2. The molar mass of lysine is 150 g/mol. Dete ...
File
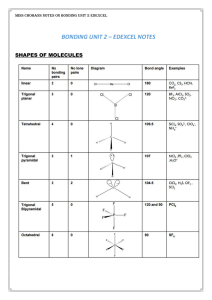
... Ionic and covalent bonding are the extremes of a continuum of bonding type. Differences in electronegativity between elements can determine where a compound lies on this scale A compound containing elements of similar electronegativity and hence a small electronegativity difference will be purely co ...
... Ionic and covalent bonding are the extremes of a continuum of bonding type. Differences in electronegativity between elements can determine where a compound lies on this scale A compound containing elements of similar electronegativity and hence a small electronegativity difference will be purely co ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.