P1_8 Muonic Atoms - Department of Physics and Astronomy
... positive nucleus) is taken as 1.672621777 , since these are the current recommended values [4]. Substituting the appropriate values into Eq. 2 yields a reduced mass of a muon of 186 times larger than the reduced mass of an electron. On substituting electrons for muons, the only parameter that change ...
... positive nucleus) is taken as 1.672621777 , since these are the current recommended values [4]. Substituting the appropriate values into Eq. 2 yields a reduced mass of a muon of 186 times larger than the reduced mass of an electron. On substituting electrons for muons, the only parameter that change ...
General Chemistry - Valdosta State University
... exactly, now we use the wavefunction(y). Wavefunction (y) – A mathematical expression to describe the shape and energy of an electron in an orbit. - The probability of finding an electron at a point in space is determined by taking the square of the wavefunction: Probability density = y2 Chapter 7 ...
... exactly, now we use the wavefunction(y). Wavefunction (y) – A mathematical expression to describe the shape and energy of an electron in an orbit. - The probability of finding an electron at a point in space is determined by taking the square of the wavefunction: Probability density = y2 Chapter 7 ...
Chapter 5 PowerPoint
... of atoms. Three rules tell us how: 1) Aufbau principle - electrons enter the lowest energy first. • This causes difficulties because of the overlap of orbitals of different energies – follow the diagram! 2) Pauli Exclusion Principle - at most 2 electrons per orbital - different spins ...
... of atoms. Three rules tell us how: 1) Aufbau principle - electrons enter the lowest energy first. • This causes difficulties because of the overlap of orbitals of different energies – follow the diagram! 2) Pauli Exclusion Principle - at most 2 electrons per orbital - different spins ...
Chapter 11
... Quanta - the amount of energy needed to move from one energy level to another. Quantum leap in energy. Schrödinger derived an equation that described the energy and position of the electrons in an atom Treated electrons as waves ...
... Quanta - the amount of energy needed to move from one energy level to another. Quantum leap in energy. Schrödinger derived an equation that described the energy and position of the electrons in an atom Treated electrons as waves ...
ATOMIC STRUCTURE
... Gram Atomic Mass. A sample of an element with amass in grams numerically equal to the atomic mass is a gram atomic mass. For example, 12 grams of carbon, 16 grams of oxygen, and 32 grams of sulfur are equal to I gram atomic mass of each element. One gram atomic mass of an element contains 6.02 x 102 ...
... Gram Atomic Mass. A sample of an element with amass in grams numerically equal to the atomic mass is a gram atomic mass. For example, 12 grams of carbon, 16 grams of oxygen, and 32 grams of sulfur are equal to I gram atomic mass of each element. One gram atomic mass of an element contains 6.02 x 102 ...
4.4 The Bohr Atom
... “What he saw was that the set of allowed frequencies (proportional to inverse wavelengths) emitted by the hydrogen atom could all be expressed as differences. This immediately suggested to him a generalization of his idea of a "stationary state" lowest energy level, in which the electron did not ra ...
... “What he saw was that the set of allowed frequencies (proportional to inverse wavelengths) emitted by the hydrogen atom could all be expressed as differences. This immediately suggested to him a generalization of his idea of a "stationary state" lowest energy level, in which the electron did not ra ...
Review Chemistry KEY - cms16-17
... 32. List each element in the following compounds and the number of atoms of each element present and the total number of atoms. a. C6H8O6 (Vitamin C): i. Elements: C, H, and O_____________________________________ ii. Atoms: C=6, H=8, and O=6 Total number of atoms=20___________ b. C8H10O2N4H2O (Caffe ...
... 32. List each element in the following compounds and the number of atoms of each element present and the total number of atoms. a. C6H8O6 (Vitamin C): i. Elements: C, H, and O_____________________________________ ii. Atoms: C=6, H=8, and O=6 Total number of atoms=20___________ b. C8H10O2N4H2O (Caffe ...
The buoyant force on an object totally submerged in a fluid depends
... Predicts emission and absorption lines of hydrogen and hydrogen-like ions Predicts x-ray emissions (Moseley’s law) Gives an intuitive picture of what goes on in an atom The correspondence principle is obeyed... sort of It can’t easily be extended to more complicated atoms No prediction of rates, lin ...
... Predicts emission and absorption lines of hydrogen and hydrogen-like ions Predicts x-ray emissions (Moseley’s law) Gives an intuitive picture of what goes on in an atom The correspondence principle is obeyed... sort of It can’t easily be extended to more complicated atoms No prediction of rates, lin ...
Chapter 9d Introduction to Quantum Mechanics
... space between two rigid reflecting walls but in three dimensional space. For hydrogen atom, a central proton holds the relatively light electron within a region of space whose dimension is of order of 0.1nm. ...
... space between two rigid reflecting walls but in three dimensional space. For hydrogen atom, a central proton holds the relatively light electron within a region of space whose dimension is of order of 0.1nm. ...
Energy levels, photons and spectral lines
... Niels Bohr developed a model of the atom where the electrons had certain stable states that had quantized radii and energy ...
... Niels Bohr developed a model of the atom where the electrons had certain stable states that had quantized radii and energy ...
O Strong-Arming Electron Spin Dynamics
... proposed using the spin of a single electron as a quantum bit. At the time of the proposal, it was not possible to trap a single electron in a device and measure its spin, let alone demonstrate control of quantum coherence. In this talk I will describe recent progress in the field, focusing on two n ...
... proposed using the spin of a single electron as a quantum bit. At the time of the proposal, it was not possible to trap a single electron in a device and measure its spin, let alone demonstrate control of quantum coherence. In this talk I will describe recent progress in the field, focusing on two n ...
Energy level - Spring-Ford Area School District
... of atoms. Three rules tell us how: 1) Aufbau principle - electrons enter the lowest energy first. • This causes difficulties because of the overlap of orbitals of different energies – follow the diagram! 2) Pauli Exclusion Principle - at most 2 electrons per orbital - different spins ...
... of atoms. Three rules tell us how: 1) Aufbau principle - electrons enter the lowest energy first. • This causes difficulties because of the overlap of orbitals of different energies – follow the diagram! 2) Pauli Exclusion Principle - at most 2 electrons per orbital - different spins ...
Chapter 5 Electrons in Atoms
... of atoms. Three rules tell us how: 1) Aufbau principle - electrons enter the lowest energy first. • This causes difficulties because of the overlap of orbitals of different energies – follow the diagram! 2) Pauli Exclusion Principle - at most 2 electrons per orbital - different spins ...
... of atoms. Three rules tell us how: 1) Aufbau principle - electrons enter the lowest energy first. • This causes difficulties because of the overlap of orbitals of different energies – follow the diagram! 2) Pauli Exclusion Principle - at most 2 electrons per orbital - different spins ...
Chapter 2
... b) is found only in molecules containing oxygen c) shares electrons equally between atoms d) ionizes e) has shared electrons pulled closer to the more electronegative atom 15. When the proton number and electron number are unequal, the atom or molecule _____. (Concept 2.3 ) a) forms a covalent bond ...
... b) is found only in molecules containing oxygen c) shares electrons equally between atoms d) ionizes e) has shared electrons pulled closer to the more electronegative atom 15. When the proton number and electron number are unequal, the atom or molecule _____. (Concept 2.3 ) a) forms a covalent bond ...
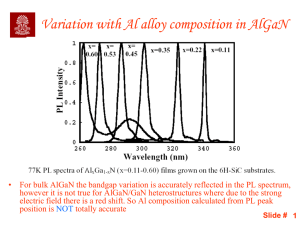
Slide 1
... With increasing well width – The intensity increases due to increased confinement – The peak position shifts to lower energy due to reduction in quantum size effect (QSE) i.e. splitting of energy levels in a QW Slide # 3 – The full width at half maximum (FWHM) also decreases ...
... With increasing well width – The intensity increases due to increased confinement – The peak position shifts to lower energy due to reduction in quantum size effect (QSE) i.e. splitting of energy levels in a QW Slide # 3 – The full width at half maximum (FWHM) also decreases ...
1. dia
... Quantum numbers describe values of conserved quantities in the dynamics of the quantum system. They often describe specifically the energies of electrons in atoms, but other possibilities include angular momentum, spin etc. It is already known from the Bohr’s atom model that the energy of the electr ...
... Quantum numbers describe values of conserved quantities in the dynamics of the quantum system. They often describe specifically the energies of electrons in atoms, but other possibilities include angular momentum, spin etc. It is already known from the Bohr’s atom model that the energy of the electr ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.