Chapter 1: Fundamental Concepts
... • Polar or Non-Polar? – In very symmetrical structures (e.g. CO2 or CF4), the individual bond dipoles effectively cancel each other and the molecule is ...
... • Polar or Non-Polar? – In very symmetrical structures (e.g. CO2 or CF4), the individual bond dipoles effectively cancel each other and the molecule is ...
L 35 Modern Physics [1] - University of Iowa Physics
... Problems with Newton’s Laws • Newton’s laws, which were so successful in allowing us to understand the behavior of big objects such as the motions of the planets, failed when pushed to explain atomic size phenomena. • The discovery of the laws of atomic physics led to every important 20th century d ...
... Problems with Newton’s Laws • Newton’s laws, which were so successful in allowing us to understand the behavior of big objects such as the motions of the planets, failed when pushed to explain atomic size phenomena. • The discovery of the laws of atomic physics led to every important 20th century d ...
L 35 Modern Physics [1] Modern Physics
... in allowing us to understand the behavior of big objects such as the motions of the planets, failed when pushed to explain atomic size phenomena. • The discovery of the laws of atomic physics led to every important 20th century discovery that have transformed our lives, the electronic revolution. ...
... in allowing us to understand the behavior of big objects such as the motions of the planets, failed when pushed to explain atomic size phenomena. • The discovery of the laws of atomic physics led to every important 20th century discovery that have transformed our lives, the electronic revolution. ...
The Quantum Mechanics of MRI
... • Pauli’s exclusion principle ensures that many shells are filled. • Nuclei with uneven (even) atomic number have half-integer (integer) spin • Nuclei with even atomic and mass numbers have zero spin. • Unpaired neutrons/protons provide the spin for MRI. ...
... • Pauli’s exclusion principle ensures that many shells are filled. • Nuclei with uneven (even) atomic number have half-integer (integer) spin • Nuclei with even atomic and mass numbers have zero spin. • Unpaired neutrons/protons provide the spin for MRI. ...
> >
... Different types of solids (metallic, ionic, covalent network, molecular) Chapter 21 - Nuclear ...
... Different types of solids (metallic, ionic, covalent network, molecular) Chapter 21 - Nuclear ...
File - Get Involved!
... periodic trends, etc at the start of the section that may help you work through some of the problems ...
... periodic trends, etc at the start of the section that may help you work through some of the problems ...
Key Concept 1: An atom is the smallest unit of an element that
... Key Concept 7: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. ...
... Key Concept 7: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. ...
key concepts of matter
... protons located in its nucleus. Key Concept 2: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. Key Concept 3: Electrons located in the outermost shell of the electron cloud are ...
... protons located in its nucleus. Key Concept 2: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. Key Concept 3: Electrons located in the outermost shell of the electron cloud are ...
PES Topography - Materials Computation Center
... One CBF per occupied orbital on an atom E.g., H has one s function, C has 2s and 1p n-zeta n CBF per occupied orbital on an atom Valence n-zeta MBS for core (1s of C), n-zeta for valence Polarized Add higher angular momentum functions than MBS – e.g., d functions on C Diffuse or augmented Add much w ...
... One CBF per occupied orbital on an atom E.g., H has one s function, C has 2s and 1p n-zeta n CBF per occupied orbital on an atom Valence n-zeta MBS for core (1s of C), n-zeta for valence Polarized Add higher angular momentum functions than MBS – e.g., d functions on C Diffuse or augmented Add much w ...
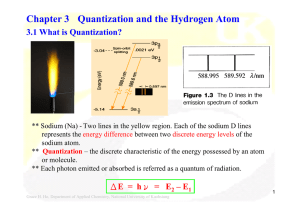
Chapter 3 Quantization and the Hydrogen Atom
... ** For more than one electron in an atom, the Schrödinger equation is no longer exactly soluble: approximations must be made. (Coulombic attraction + electron-electron repulsion) ** The (2l + 1) degeneracy of the s, p, d, … orbitals is removed. ** Aufbau principle – Electrons are fed into the availa ...
... ** For more than one electron in an atom, the Schrödinger equation is no longer exactly soluble: approximations must be made. (Coulombic attraction + electron-electron repulsion) ** The (2l + 1) degeneracy of the s, p, d, … orbitals is removed. ** Aufbau principle – Electrons are fed into the availa ...
File
... compounds into oxygen molecules and hydrogen molecules Water Oxygen + Hydrogen H2O O2 +H2 The electrolysis reaction proves that compounds are made of more than one kind of element. Dalton’s Atomic Theory: 1. All matter is made up of small particles called atoms 2. Atoms can’t be created or des ...
... compounds into oxygen molecules and hydrogen molecules Water Oxygen + Hydrogen H2O O2 +H2 The electrolysis reaction proves that compounds are made of more than one kind of element. Dalton’s Atomic Theory: 1. All matter is made up of small particles called atoms 2. Atoms can’t be created or des ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.


![L 35 Modern Physics [1] - University of Iowa Physics](http://s1.studyres.com/store/data/000679677_1-b925cf8c8f031b0f2b0c09a806312d20-300x300.png)
![L 35 Modern Physics [1] Modern Physics](http://s1.studyres.com/store/data/001558975_1-84d6e03bc786b63795533f59711ce2f4-300x300.png)