1.1 Materials Self
... The confinement can be due to (1) electrostatic potentials (generated by external electrodes, doping, strain, impurities), (2) the presence of an interface between different semiconductor materials (e.g., in the case of self-assembled quantum dots), (3) the presence of the semiconductor surface (e.g ...
... The confinement can be due to (1) electrostatic potentials (generated by external electrodes, doping, strain, impurities), (2) the presence of an interface between different semiconductor materials (e.g., in the case of self-assembled quantum dots), (3) the presence of the semiconductor surface (e.g ...
Chapter 9 Molecular Geometry and Bonding Theories
... The HF molecule has a bond dipole – a charge separation due to the electronegativity difference between F and H. The shape of a molecule and the magnitude of the bond dipole(s) can give the molecule an overall degree of polarity dipole moment. ...
... The HF molecule has a bond dipole – a charge separation due to the electronegativity difference between F and H. The shape of a molecule and the magnitude of the bond dipole(s) can give the molecule an overall degree of polarity dipole moment. ...
form revision a
... Check your key area statements. If not green you need to do more work! Knowledge of the structure of the periodic table, groups and periods. All matter is made of atoms. When a substance contains only one kind of atom it is known as an element. Atoms contain protons, neutrons and electrons each with ...
... Check your key area statements. If not green you need to do more work! Knowledge of the structure of the periodic table, groups and periods. All matter is made of atoms. When a substance contains only one kind of atom it is known as an element. Atoms contain protons, neutrons and electrons each with ...
Ch 4 Review
... ____ 44. molecule ____ 45. compound ____ 46. electron ____ 47. mass number ____ 48. atomic number ____ 49. ion Match each item with the correct statement below. a. the number of protons plus the number of neutrons in an atom b. an atom or molecule that has gained or lost one or more electrons c. th ...
... ____ 44. molecule ____ 45. compound ____ 46. electron ____ 47. mass number ____ 48. atomic number ____ 49. ion Match each item with the correct statement below. a. the number of protons plus the number of neutrons in an atom b. an atom or molecule that has gained or lost one or more electrons c. th ...
Objective 4
... full of puppies who have plenty of bones to go around and are not possessive of any one particular bone. This allows the electrons to move through the substance with little ...
... full of puppies who have plenty of bones to go around and are not possessive of any one particular bone. This allows the electrons to move through the substance with little ...
Introduction to elementary quantum mechanics
... micrometer. As classical systems we can consider individual objects having macroscopic masses and sizes (usually modeled as material points) or sets of such objects (e.g. planetary systems). The aim of theory is the description of the state of a physical system. In classical mechanics the state of a ...
... micrometer. As classical systems we can consider individual objects having macroscopic masses and sizes (usually modeled as material points) or sets of such objects (e.g. planetary systems). The aim of theory is the description of the state of a physical system. In classical mechanics the state of a ...
The Wave
... 1. EM radiation of frequency 7.0 X 1014 Hz falls on a metal with work function of 0.5eV. a) Calculate the maximum kinetic energy of the emitted photoelectrons and the maximum speed of the emitted photoelectrons. ...
... 1. EM radiation of frequency 7.0 X 1014 Hz falls on a metal with work function of 0.5eV. a) Calculate the maximum kinetic energy of the emitted photoelectrons and the maximum speed of the emitted photoelectrons. ...
PHY 104: Modern Physics - Physlab
... why are some materials hard and others soft, why do metals, for example, conduct electricity and heat easily, while glass doesn’t. Quantum physics also forms the basis of our understanding of the chemical world, materials science, as well as electronic devices permeating the modern digital age. The ...
... why are some materials hard and others soft, why do metals, for example, conduct electricity and heat easily, while glass doesn’t. Quantum physics also forms the basis of our understanding of the chemical world, materials science, as well as electronic devices permeating the modern digital age. The ...
Matter Waves and Obital Quantum Numbers
... Matter Waves and Orbital Quantum Numbers Roger Ellman In general the 20th Century concept of the overall arrangement of atom's orbital electrons developed as follows. - The electron orbits are located in shells, a shell conceptually being a spherical surface with the atomic nucleus located at the c ...
... Matter Waves and Orbital Quantum Numbers Roger Ellman In general the 20th Century concept of the overall arrangement of atom's orbital electrons developed as follows. - The electron orbits are located in shells, a shell conceptually being a spherical surface with the atomic nucleus located at the c ...
NYS Regents Chemistry June 21, 2002
... June 21, 2002 1. What is the electron configuration of a sulfur atom in the ground state? (A) 2–4 (C) 2–8–4 (B) 2–6 (D) 2–8–6 ...
... June 21, 2002 1. What is the electron configuration of a sulfur atom in the ground state? (A) 2–4 (C) 2–8–4 (B) 2–6 (D) 2–8–6 ...
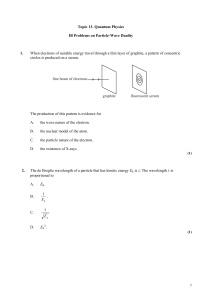
Wave-particle_duality
... Determine the wavelength associated with the electrons as predicted by the de Broglie hypothesis. ...
... Determine the wavelength associated with the electrons as predicted by the de Broglie hypothesis. ...
ChemFinalgeocities
... If 9.0 g of water contain 1.0 g of hydrogen, what mass of oxygen is contained in 36 g of water? a. 4.0 g c. 10.0 g b. 8.0 g d. 32 g Which of the following statements is not a main point of Dalton's atomic theory? a. All matter is made up of atoms. b. Atoms are made up of smaller particles. c. Atoms ...
... If 9.0 g of water contain 1.0 g of hydrogen, what mass of oxygen is contained in 36 g of water? a. 4.0 g c. 10.0 g b. 8.0 g d. 32 g Which of the following statements is not a main point of Dalton's atomic theory? a. All matter is made up of atoms. b. Atoms are made up of smaller particles. c. Atoms ...
Unit 1 – Physical Science and Chemical Reactions
... The mass of the protons was too small to account for the total atomic mass of the atom so Rutherford predicted that there must be a neutral particle in the nucleus similar in mass to the proton In 1932 James Chadwick demonstrated this particle, which he called a neutron. (He was the first to nam ...
... The mass of the protons was too small to account for the total atomic mass of the atom so Rutherford predicted that there must be a neutral particle in the nucleus similar in mass to the proton In 1932 James Chadwick demonstrated this particle, which he called a neutron. (He was the first to nam ...
Chapter 2
... Atomic structure determines the behavior of an element (pp. 28-33, FIGURE 2.10) An atom is the smallest unit of an element. An atom has a nucleus made up of positively charged protons and uncharged neutrons, as well as a surrounding cloud of negatively charged electrons. The number of electrons in ...
... Atomic structure determines the behavior of an element (pp. 28-33, FIGURE 2.10) An atom is the smallest unit of an element. An atom has a nucleus made up of positively charged protons and uncharged neutrons, as well as a surrounding cloud of negatively charged electrons. The number of electrons in ...
AP Biology
... Atomic structure determines the behavior of an element (pp. 28-33, FIGURE 2.10) An atom is the smallest unit of an element. An atom has a nucleus made up of positively charged protons and uncharged neutrons, as well as a surrounding cloud of negatively charged electrons. The number of electrons in ...
... Atomic structure determines the behavior of an element (pp. 28-33, FIGURE 2.10) An atom is the smallest unit of an element. An atom has a nucleus made up of positively charged protons and uncharged neutrons, as well as a surrounding cloud of negatively charged electrons. The number of electrons in ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.