Newton’s first law
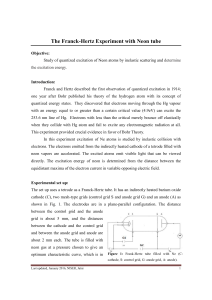
... move to the plate and the current rises accordingly. For mercury atoms, when V=4.9V, the electrons make inelastic collision and leave the atom jump to a high orbit (n=2). The original electrons move off with little energy and could not reach the plate and thus reduce the current. As V is increas ...
... move to the plate and the current rises accordingly. For mercury atoms, when V=4.9V, the electrons make inelastic collision and leave the atom jump to a high orbit (n=2). The original electrons move off with little energy and could not reach the plate and thus reduce the current. As V is increas ...
1.3.5 Spectroscopy Name Symbol Definition SI unit Notes total term
... atoms). If the value of Ω is not specified, the term symbols is taken to refer to all component states, and a right subscript r or i may be added to indicate that the components are regular (energy increases with Ω) or inverted (energy decreases with Ω) respectively. The electronic states of molecul ...
... atoms). If the value of Ω is not specified, the term symbols is taken to refer to all component states, and a right subscript r or i may be added to indicate that the components are regular (energy increases with Ω) or inverted (energy decreases with Ω) respectively. The electronic states of molecul ...
Chapter 9: Molecular Geometry and Hybridization of Atomic Orbitals
... This will result in two non-equivalent Be-Cl bonds. However, experiments suggest that the two Be-Cl bonds are equivalent in every respect. Thus, the 2s and the 2p orbitals in the Be-atom must be hybridized to form two equivalent sp hybrid orbitals. The two hybrid orbitals lie on the same axi ...
... This will result in two non-equivalent Be-Cl bonds. However, experiments suggest that the two Be-Cl bonds are equivalent in every respect. Thus, the 2s and the 2p orbitals in the Be-atom must be hybridized to form two equivalent sp hybrid orbitals. The two hybrid orbitals lie on the same axi ...
Chapter 7
... Microscopic dynamics: Quantum mechanics • Classical physics failed to account for the existence of discrete energies of atoms and other experiments in the early 20th century. • Such total failures show that the basic concept of classical mechanics need to be corrected fundamentally. • A new mechani ...
... Microscopic dynamics: Quantum mechanics • Classical physics failed to account for the existence of discrete energies of atoms and other experiments in the early 20th century. • Such total failures show that the basic concept of classical mechanics need to be corrected fundamentally. • A new mechani ...
PHYS1220 - s3.amazonaws.com
... position and momentum of an electron as precisely as possible with a powerful light microscope In order to determine the electron’s location (ie making x small ~ l) at least one photon of light (with momentum h/l must be scattered (as in (a)) But the photon imparts an unknown amount of its momentum ...
... position and momentum of an electron as precisely as possible with a powerful light microscope In order to determine the electron’s location (ie making x small ~ l) at least one photon of light (with momentum h/l must be scattered (as in (a)) But the photon imparts an unknown amount of its momentum ...
Year End Chemistry Review
... 6. Periodic Table, periods and group names 7. Periodic trends: (ionization energy, electron affinity, electronegativity, atomic radius) 8. Atomic number = # of _____ Mass number = # of ________ Isotopes are atoms of the same element, therefore having the same number of __________, but different numb ...
... 6. Periodic Table, periods and group names 7. Periodic trends: (ionization energy, electron affinity, electronegativity, atomic radius) 8. Atomic number = # of _____ Mass number = # of ________ Isotopes are atoms of the same element, therefore having the same number of __________, but different numb ...
Quantum Chemistry and Spectroscopy
... compounds are transparent. And UV radiation, especially strong UV, will broke most molecules. (Visible light 400 -700 nm.) The table contain the lowest excitation and there are other transitions higher in energy. The chromophores below are simple but nature is full of more complex chromophores. One ...
... compounds are transparent. And UV radiation, especially strong UV, will broke most molecules. (Visible light 400 -700 nm.) The table contain the lowest excitation and there are other transitions higher in energy. The chromophores below are simple but nature is full of more complex chromophores. One ...
Part II - Web site of Dr. Charles Berks
... In order to appreciate the Born-Haber cycle we must review the concept of enthalpy of formation. By definition, the enthalpy of formation is the enthalpy change associated with the formation of 1 mole of a chemical in a particular state from its elements in their standard states. The enthalpy of fo ...
... In order to appreciate the Born-Haber cycle we must review the concept of enthalpy of formation. By definition, the enthalpy of formation is the enthalpy change associated with the formation of 1 mole of a chemical in a particular state from its elements in their standard states. The enthalpy of fo ...
Document
... distance the orbit was from the nucleus. • Electrons emit radiation when they “jump” from an orbit with higher energy down to an orbit with lower energy. – The emitted radiation was a photon of light. – The distance between the orbits determined the energy of the photon of light produced. © 2014 Pea ...
... distance the orbit was from the nucleus. • Electrons emit radiation when they “jump” from an orbit with higher energy down to an orbit with lower energy. – The emitted radiation was a photon of light. – The distance between the orbits determined the energy of the photon of light produced. © 2014 Pea ...
Introduction to Atomic Spectroscopy
... When gaseous atoms at high temperatures are irradiated with a monochromatic beam of radiation of enough energy to cause electronic excitation, emission takes place in all directions. The emitted radiation from the first excited electronic level, collected at 90o to the incident beam, is called reson ...
... When gaseous atoms at high temperatures are irradiated with a monochromatic beam of radiation of enough energy to cause electronic excitation, emission takes place in all directions. The emitted radiation from the first excited electronic level, collected at 90o to the incident beam, is called reson ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.