Chapter 1 Assignment Section 1.1 1. Why is air classified as matter
... 3. Why would you expect Li and S to have different chemical and physical properties? Section 6.3 1. Why is a magnesium atom smaller than a sodium atom and a calcium atom? 2. Would you expect a Cl― ion to be larger or smaller than Mg2+? 3. Which would have a larger first ionization energy? a) Na or L ...
... 3. Why would you expect Li and S to have different chemical and physical properties? Section 6.3 1. Why is a magnesium atom smaller than a sodium atom and a calcium atom? 2. Would you expect a Cl― ion to be larger or smaller than Mg2+? 3. Which would have a larger first ionization energy? a) Na or L ...
AP Unit 0: Chemical Foundations
... ◦ Hold molecules/atoms as close together as possible ◦ Hold a shape ...
... ◦ Hold molecules/atoms as close together as possible ◦ Hold a shape ...
Cold encounters: Electrons and molecules
... overlap is zero. But let us say that the molecule borrows some time from the classically inaccessible quantum world, in a manner restricted by the Heisenberg relationship Llli.~12h/27t. In this borrowed time, the system may explore paths in which the molecule is "virtually bent", where virtual impli ...
... overlap is zero. But let us say that the molecule borrows some time from the classically inaccessible quantum world, in a manner restricted by the Heisenberg relationship Llli.~12h/27t. In this borrowed time, the system may explore paths in which the molecule is "virtually bent", where virtual impli ...
the tasks for those beginning
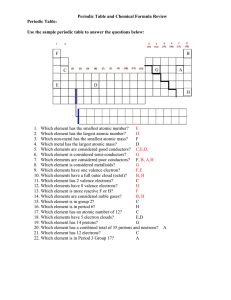
... Chemistry topic 1 – Electronic structure, how electrons are arranged around the nucleus A periodic table can give you the proton / atomic number of an element, this also tells you how many electrons are in the atom. You will have used the rule of electrons shell filling, where: The first shell holds ...
... Chemistry topic 1 – Electronic structure, how electrons are arranged around the nucleus A periodic table can give you the proton / atomic number of an element, this also tells you how many electrons are in the atom. You will have used the rule of electrons shell filling, where: The first shell holds ...
L14special - Particle Physics and Particle Astrophysics
... For bound states the wavefunction penetrates the classically forbidden region. Thus, the particle exists in a region where its kinetic energy is negative. To find energies of these states we’ll solve the time independent Schrödinger equation: ...
... For bound states the wavefunction penetrates the classically forbidden region. Thus, the particle exists in a region where its kinetic energy is negative. To find energies of these states we’ll solve the time independent Schrödinger equation: ...
Chapter 28: Problems
... Finally, the spin angular momentum can take on one of only two values, conventionally referred to as “spin up” and “spin down.” The spin angular momentum is characterized by the spin quantum number, which can take on values of +1/2 or –1/2. Understanding the periodic table of elements One key to und ...
... Finally, the spin angular momentum can take on one of only two values, conventionally referred to as “spin up” and “spin down.” The spin angular momentum is characterized by the spin quantum number, which can take on values of +1/2 or –1/2. Understanding the periodic table of elements One key to und ...
1 - 嘉義大學
... 11. What is the wavelength of a photon of red light (in nm) whose frequency is 4.60 ×1014 Hz? (A) 652 nm (B) 153×106 nm (C) 153 nm (D) 460 nm 12. Which of the following is not determined by the principal quantum number, n, of the electron in a hydrogen atom? (A) the energy of the electron (B) the mi ...
... 11. What is the wavelength of a photon of red light (in nm) whose frequency is 4.60 ×1014 Hz? (A) 652 nm (B) 153×106 nm (C) 153 nm (D) 460 nm 12. Which of the following is not determined by the principal quantum number, n, of the electron in a hydrogen atom? (A) the energy of the electron (B) the mi ...
phys3313-fall12
... • The mean kinetic energy of the electron-nucleus system is quantized as K = nhforb/2, where forb is the frequency of rotation. This is equivalent to the angular momentum of a stationary state to be an integral multiple of h/2 Wednesday, Sept. 26, ...
... • The mean kinetic energy of the electron-nucleus system is quantized as K = nhforb/2, where forb is the frequency of rotation. This is equivalent to the angular momentum of a stationary state to be an integral multiple of h/2 Wednesday, Sept. 26, ...
ME 533 Lecture 6 Pla..
... • Classification of electronically, excited states of diatomic and linear polyatomic molecules is somewhat similar to atoms. • quantum number Λ =0,1,2,3 (corresponding Greek symbols Σ, Π, Δ, Φ), describes the absolute value of the component of the total orbital angular momentum along the internuclea ...
... • Classification of electronically, excited states of diatomic and linear polyatomic molecules is somewhat similar to atoms. • quantum number Λ =0,1,2,3 (corresponding Greek symbols Σ, Π, Δ, Φ), describes the absolute value of the component of the total orbital angular momentum along the internuclea ...
1. All matter is made up of
... a. I am a member of the Boron family with 49 protons. b. I have a total of 74 electrons in an atom. c. I have an atomic mass of ...
... a. I am a member of the Boron family with 49 protons. b. I have a total of 74 electrons in an atom. c. I have an atomic mass of ...
1. Review (MC problems, due Monday) 2. - mvhs
... 3. A solution of barium hydroxide is titrated with 0.1-M sulfuric acid and the electrical conductivity of the solution is measured as the titration proceeds. a) For the reaction that occurs during the titration described above, write a balanced net ionic equation. (b) Explain why the conductivity de ...
... 3. A solution of barium hydroxide is titrated with 0.1-M sulfuric acid and the electrical conductivity of the solution is measured as the titration proceeds. a) For the reaction that occurs during the titration described above, write a balanced net ionic equation. (b) Explain why the conductivity de ...
Assignment 8 - Duke Physics
... The balloon-like shapes represent surfaces of large electron probability density |Ψ|2 where the electron in that orbital has a large probability of being found inside the balloon; we will learn later in this course how to calculate these shapes mathematically when we solve the Schrodinger equation f ...
... The balloon-like shapes represent surfaces of large electron probability density |Ψ|2 where the electron in that orbital has a large probability of being found inside the balloon; we will learn later in this course how to calculate these shapes mathematically when we solve the Schrodinger equation f ...
6.1 The Waves Nature of Light
... 6.5 Quantum Mechanics & Atomic Orbitals • Erwin Schrödinger’s mathematical treatment : both the wave and particle nature of matter could be incorporated. quantum mechanics. : wave equation 2 : gives a probability density map of where an electron has a certain statistical likelihood of being at ...
... 6.5 Quantum Mechanics & Atomic Orbitals • Erwin Schrödinger’s mathematical treatment : both the wave and particle nature of matter could be incorporated. quantum mechanics. : wave equation 2 : gives a probability density map of where an electron has a certain statistical likelihood of being at ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.