Particle Identification in High Energy Physics
... Example: Fermilab Tevatron ring: p≈2 TeV/c = 106 MeV/c, superconducting magnets produce B=4.2 Tesla = 42000 Gauss r = 79,365 cm = 0.794 km Divide r by 2 if the particle has charge 2e... ...
... Example: Fermilab Tevatron ring: p≈2 TeV/c = 106 MeV/c, superconducting magnets produce B=4.2 Tesla = 42000 Gauss r = 79,365 cm = 0.794 km Divide r by 2 if the particle has charge 2e... ...
Honors Chemistry Name_________________________________
... Explain through emission spectra that electronic energy levels are quantized. Calculate the difference of energy between two levels given the wavelength or frequency of light emitted. Recognize the basic shapes of s, p and d orbitals. Describe atomic orbitals in a multielectron atom in terms ...
... Explain through emission spectra that electronic energy levels are quantized. Calculate the difference of energy between two levels given the wavelength or frequency of light emitted. Recognize the basic shapes of s, p and d orbitals. Describe atomic orbitals in a multielectron atom in terms ...
CHAPTER 5 Carrier Transport Phenomena
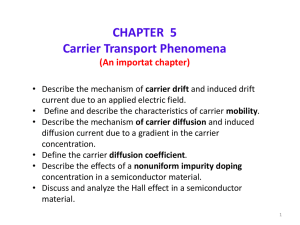
... • Describe the effects of a nonuniform impurity doping concentration in a semiconductor material. • Discuss and analyze the Hall effect in a semiconductor material. ...
... • Describe the effects of a nonuniform impurity doping concentration in a semiconductor material. • Discuss and analyze the Hall effect in a semiconductor material. ...
[a,b]! - Nikhef
... How do you avoid that all particles tumble into the negative energy levels? Simple: assume that all negative energy levels are filled (possible thanks to Pauli exclusion principle!) E=0 ...
... How do you avoid that all particles tumble into the negative energy levels? Simple: assume that all negative energy levels are filled (possible thanks to Pauli exclusion principle!) E=0 ...
Classification of the Elementary Particles
... the mesic orbit has a very small radius compared with the electron orbit, as can be seen from the formula for the first Bohr radius ...
... the mesic orbit has a very small radius compared with the electron orbit, as can be seen from the formula for the first Bohr radius ...
Notes-15 - KSU Physics
... If both electrons are excited, such as 2s3p or 3p4d, then these states are doubly excited states, since both electrons are excited. These doubly excited states have binding energies higher than the single ionization energy. Therefore these doubly excited states are not stable and they are called au ...
... If both electrons are excited, such as 2s3p or 3p4d, then these states are doubly excited states, since both electrons are excited. These doubly excited states have binding energies higher than the single ionization energy. Therefore these doubly excited states are not stable and they are called au ...
Modern Physics Homework
... Ultraviolet light of wavelength 350 nm and intensity 1.00 W/m2 is directed at a potassium surface (work function 2.2 eV). a) Find the maximum kinetic energy of the photoelectrons. b) If 0.5 percent of the incident photons produce photoelectrons, how many photoelectrons are emitted per second if the ...
... Ultraviolet light of wavelength 350 nm and intensity 1.00 W/m2 is directed at a potassium surface (work function 2.2 eV). a) Find the maximum kinetic energy of the photoelectrons. b) If 0.5 percent of the incident photons produce photoelectrons, how many photoelectrons are emitted per second if the ...
Atomic Diffraction Dr. Janine Shertzer College of the Holy Cross
... The wave-particle duality is fundamental to quantum mechanics. Light can behave like a particle (photon); matter can behave like a wave. The wavelength associated with a particle is inversely proportional to its momentum p: λ = h / p, where h is Planck’s constant. For cold atoms, the wavelength is l ...
... The wave-particle duality is fundamental to quantum mechanics. Light can behave like a particle (photon); matter can behave like a wave. The wavelength associated with a particle is inversely proportional to its momentum p: λ = h / p, where h is Planck’s constant. For cold atoms, the wavelength is l ...
Mass and Energy
... MASS and REST MASS In 1905 Einstein showed that the mass of a moving object, as measured by a stationary observer, increases as it approaches the speed of light. This effect is important for fundamental particles which can be accelerated to very high velocities.Therefore we use the term rest mass ( ...
... MASS and REST MASS In 1905 Einstein showed that the mass of a moving object, as measured by a stationary observer, increases as it approaches the speed of light. This effect is important for fundamental particles which can be accelerated to very high velocities.Therefore we use the term rest mass ( ...
Interaction of Radiation with Matter
... radiation or EMR) is a form of energy emitted and absorbed by charged particles which exhibits wave-like behavior as it travels through space. EMR has both electric and magnetic field components. In a vacuum, electromagnetic radiation propagates at a characteristic speed, the speed of light. ...
... radiation or EMR) is a form of energy emitted and absorbed by charged particles which exhibits wave-like behavior as it travels through space. EMR has both electric and magnetic field components. In a vacuum, electromagnetic radiation propagates at a characteristic speed, the speed of light. ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... (this micro wave transition may be used also for atomic clocks). Assume that the maximum JZ of the higher level is 2.5 and for the lower level its 1.5. What will the photon spectrum look like (assume that a photon can only change JZ by 0 or +/-1). Answer: JZ for the higher level is -2.5, -1.5, -0.5, ...
... (this micro wave transition may be used also for atomic clocks). Assume that the maximum JZ of the higher level is 2.5 and for the lower level its 1.5. What will the photon spectrum look like (assume that a photon can only change JZ by 0 or +/-1). Answer: JZ for the higher level is -2.5, -1.5, -0.5, ...
chemistry-study-guide-grade
... Use the VSEPR model to determine the molecular shape of a molecule. Explain the difference between electron domain geometry and molecular geometry. Determine the electron domain geometry and molecular geometry. Calculate the ideal bond angles for a molecule. Determine whether a molecule will exhibit ...
... Use the VSEPR model to determine the molecular shape of a molecule. Explain the difference between electron domain geometry and molecular geometry. Determine the electron domain geometry and molecular geometry. Calculate the ideal bond angles for a molecule. Determine whether a molecule will exhibit ...
Chapter 4 Exam Review Democritus named tiny pieces of matter
... 6. During the gold foil experiment, what caused some of the alpha particles to bounce straight back from the gold foil? ______________________________________________________________ 7. The gold foil experiment provided evidence that there is a ____________, ___________________________ charged mass ...
... 6. During the gold foil experiment, what caused some of the alpha particles to bounce straight back from the gold foil? ______________________________________________________________ 7. The gold foil experiment provided evidence that there is a ____________, ___________________________ charged mass ...
Have a closer look at electron microscopy
... Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles cha ...
... Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles cha ...
UNM Physics 262, Problem Set 12, Fall 2006
... of the hydrogen atom entirely in terms of its radius. What radius corresponds to the lowest possible energy? (c) In the lowest energy quantum mechanical con guration of the hydrogen atom, the momentum of the electron (which is entirely azimuthal) and its location along the circumference of its orbit ...
... of the hydrogen atom entirely in terms of its radius. What radius corresponds to the lowest possible energy? (c) In the lowest energy quantum mechanical con guration of the hydrogen atom, the momentum of the electron (which is entirely azimuthal) and its location along the circumference of its orbit ...
12.50, £6.25. GA Jones. Pp. 175. London" Edward
... vast amount of existing information about electron microscopy and its applications, with special emphasis on the problems in solid-state physics. The resulting book contains fundamental information about the principles on which electron microscopy is based, the design and construction of electron mi ...
... vast amount of existing information about electron microscopy and its applications, with special emphasis on the problems in solid-state physics. The resulting book contains fundamental information about the principles on which electron microscopy is based, the design and construction of electron mi ...
Exam topics-- understand and be able to apply ideas like in
... 10. Potential energy of electron in atom. 11. Atomic discharge lamps- how work and why so efficient at converting electrical energy to light. 12. Bohr model. What it explained and what were its shortcomings. 13. spin and stern-gerlach experiment-- sending atoms through inhomogeneous magnetic field. ...
... 10. Potential energy of electron in atom. 11. Atomic discharge lamps- how work and why so efficient at converting electrical energy to light. 12. Bohr model. What it explained and what were its shortcomings. 13. spin and stern-gerlach experiment-- sending atoms through inhomogeneous magnetic field. ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.


![[a,b]! - Nikhef](http://s1.studyres.com/store/data/000147861_1-4659b0cc203c9fe99ee5f554409aa79c-300x300.png)