Atomic Structure Mini Lab
... Baggies contain the following: White bead represents a proton Purple bead represents a neutron Blue bead represents an electron Count the number of protons, neutrons and electrons and fill out the data table below: Results Bag # ...
... Baggies contain the following: White bead represents a proton Purple bead represents a neutron Blue bead represents an electron Count the number of protons, neutrons and electrons and fill out the data table below: Results Bag # ...
ψ 2
... atom. Almost all of the mass in an atom is made up from the protons and neutrons in the nucleus, with a very small contribution from the orbiting electrons. The diameter of the nucleus is in the range of 1.6 fm (1.6 × 10−15 m) (for a proton in light hydrogen) to about 15 fm (for the heaviest atoms, ...
... atom. Almost all of the mass in an atom is made up from the protons and neutrons in the nucleus, with a very small contribution from the orbiting electrons. The diameter of the nucleus is in the range of 1.6 fm (1.6 × 10−15 m) (for a proton in light hydrogen) to about 15 fm (for the heaviest atoms, ...
Chemistry: Matter and Change
... • Bohr’s atomic model attributes hydrogen’s emission spectrum to electrons dropping from higher-energy to lower-energy orbits. ∆E = E higher-energy orbit - E lower-energy orbit = E photon = hν • The de Broglie equation relates a particle’s wavelength to its mass, its velocity, and Planck’s constant. ...
... • Bohr’s atomic model attributes hydrogen’s emission spectrum to electrons dropping from higher-energy to lower-energy orbits. ∆E = E higher-energy orbit - E lower-energy orbit = E photon = hν • The de Broglie equation relates a particle’s wavelength to its mass, its velocity, and Planck’s constant. ...
probability = ψ 2
... atom. Almost all of the mass in an atom is made up from the protons and neutrons in the nucleus, with a very small contribution from the orbiting electrons. The diameter of the nucleus is in the range of 1.6 fm (1.6 × 10−15 m) (for a proton in light hydrogen) to about 15 fm (for the heaviest atoms, ...
... atom. Almost all of the mass in an atom is made up from the protons and neutrons in the nucleus, with a very small contribution from the orbiting electrons. The diameter of the nucleus is in the range of 1.6 fm (1.6 × 10−15 m) (for a proton in light hydrogen) to about 15 fm (for the heaviest atoms, ...
Fall Exam 3 - Chemistry - University of Kentucky
... Which one of the following is not true regarding the wave nature of light? ...
... Which one of the following is not true regarding the wave nature of light? ...
Chapter One
... example between their crests It is measured in units of distance (m, cm, Å) Frequency () is the number of wave crests passing a given point per unit time (for example, per second) It is measured in units of 1/time, usually s-1 1 s-1 = 1 Hz (Hertz) ...
... example between their crests It is measured in units of distance (m, cm, Å) Frequency () is the number of wave crests passing a given point per unit time (for example, per second) It is measured in units of 1/time, usually s-1 1 s-1 = 1 Hz (Hertz) ...
chm 1045
... electron in an atom can change energy only by going from one energy level to another energy level. By doing so, the electron undergoes a transition. An electron goes from a higher energy level (Ei) to a lower energy level (Ef) emitting light: ...
... electron in an atom can change energy only by going from one energy level to another energy level. By doing so, the electron undergoes a transition. An electron goes from a higher energy level (Ei) to a lower energy level (Ef) emitting light: ...
The Structure of the Atom
... cause. This may, for example, be the explanation of the fact that the helium atom has not quite four times the mass of the hydrogen atom. Until, however, the nucleus theory has been more definitely tested, it would appear premature to discuss the possible structure of the nucleus itself. The general ...
... cause. This may, for example, be the explanation of the fact that the helium atom has not quite four times the mass of the hydrogen atom. Until, however, the nucleus theory has been more definitely tested, it would appear premature to discuss the possible structure of the nucleus itself. The general ...
THE HISTORY OF THE ATOM Table of Contents Black Boxes
... The ancient Greeks (back around 500BC) believed there only four elements: Earth Air Water Fire ...
... The ancient Greeks (back around 500BC) believed there only four elements: Earth Air Water Fire ...
LESSON No. 2 – Structure of atom
... (7) A compound contain 4.07% hydrogen, 24.27% carbon & 71.65% chlorine,2ts molecules mars is 98.96 what are its empirical & moleculas formula. (8) Calculate the volume of oxygen at NTP that would be required to convert 5.2L of Co to Co2. (9) Calculate the mars and charge of one mole of electron. (10 ...
... (7) A compound contain 4.07% hydrogen, 24.27% carbon & 71.65% chlorine,2ts molecules mars is 98.96 what are its empirical & moleculas formula. (8) Calculate the volume of oxygen at NTP that would be required to convert 5.2L of Co to Co2. (9) Calculate the mars and charge of one mole of electron. (10 ...
Atomic Theory Notes Packet
... energy is added, the electrons are promoted to higher, or excited, energy states. As the electrons lose this energy, light is emitted. The wavelengths of the light emitted enable us to determine the energy levels that the electrons occupied. 2. This emitted light may be measured using a spectroscope ...
... energy is added, the electrons are promoted to higher, or excited, energy states. As the electrons lose this energy, light is emitted. The wavelengths of the light emitted enable us to determine the energy levels that the electrons occupied. 2. This emitted light may be measured using a spectroscope ...
Notes 10
... To find the direction of the magnetic field force employ right hand rule number 1: 1. Point fingers of right hand in the direction of the velocity v. 2. Curl fingers in the direction of the magnetic field B moving through the smallest angle. 3. Thumb is pointing in the direction of the magnetic forc ...
... To find the direction of the magnetic field force employ right hand rule number 1: 1. Point fingers of right hand in the direction of the velocity v. 2. Curl fingers in the direction of the magnetic field B moving through the smallest angle. 3. Thumb is pointing in the direction of the magnetic forc ...
Step Potential
... The penetration of the barrier is not unique to quantum mechanics. When light is totally reflected from the glass-air interface, the light wave can penetrate the air barrier if a second peace of glass is brought within a few wavelengths of the first, even when the angle of incidence in the first pr ...
... The penetration of the barrier is not unique to quantum mechanics. When light is totally reflected from the glass-air interface, the light wave can penetrate the air barrier if a second peace of glass is brought within a few wavelengths of the first, even when the angle of incidence in the first pr ...
Rotation ,vibration, electronic spectra
... two N atoms held together with a massless, rigid rod (rigid rotator model). • In a purely rotational system, the kinetic energy is expressed in terms of the angular momentum L and rotational inertia I. ...
... two N atoms held together with a massless, rigid rod (rigid rotator model). • In a purely rotational system, the kinetic energy is expressed in terms of the angular momentum L and rotational inertia I. ...
16_04_2013 - IB Phys.. - hrsbstaff.ednet.ns.ca
... • http://www.youtube.com/watch?NR=1&fea ture=fvwp&v=2xnsMGNicho ...
... • http://www.youtube.com/watch?NR=1&fea ture=fvwp&v=2xnsMGNicho ...
Bose-Einstein condensation of excitons and cold atoms OECS13
... exciton thermal De Broglie wavelength by more than an order of magnitude. The comparison of theory and experiment allows extracting the spin currents carried by electrons and holes bound to excitons. The spins tend to align along the effective spin-orbit field induced due to the Dresselhaus effect ( ...
... exciton thermal De Broglie wavelength by more than an order of magnitude. The comparison of theory and experiment allows extracting the spin currents carried by electrons and holes bound to excitons. The spins tend to align along the effective spin-orbit field induced due to the Dresselhaus effect ( ...
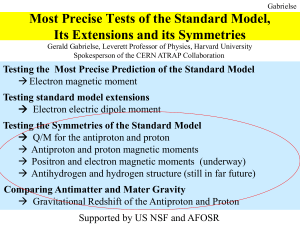
Most Precise Tests of the Standard Model, Its
... Schiff (1963) – no atomic or molecular EDM (i.e. linear Stark effect) • from electron edm • nonrelativistic quantum mechanics limit Sandars (1965) – can get atomic or molecular EDM (i.e. linear Stark effect) • from electron edm • relativistic quantum mechanics • get significant enhancement (D >> d) ...
... Schiff (1963) – no atomic or molecular EDM (i.e. linear Stark effect) • from electron edm • nonrelativistic quantum mechanics limit Sandars (1965) – can get atomic or molecular EDM (i.e. linear Stark effect) • from electron edm • relativistic quantum mechanics • get significant enhancement (D >> d) ...
STEM Fair Introduction Beanium Isotopes Lab
... Neutrons are made of one “up” quark and two “down” quarks ...
... Neutrons are made of one “up” quark and two “down” quarks ...
Animator Help Session
... have pointers to all particles and a marching variable (time) for simulation If you have two separate simulations (say, cloth sim and particles that respond to viscous drag) you may want to make that distinction here (as well as in your force and particle implementation ...
... have pointers to all particles and a marching variable (time) for simulation If you have two separate simulations (say, cloth sim and particles that respond to viscous drag) you may want to make that distinction here (as well as in your force and particle implementation ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.