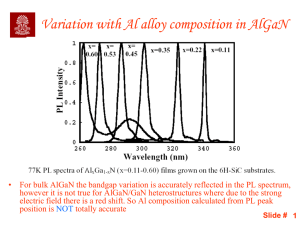
The Quantum Spin Hall Effect
... • The spin Hall effect has now been experimentally observed. (Science 2004, PRL ...
... • The spin Hall effect has now been experimentally observed. (Science 2004, PRL ...
Introduction State vectors
... • a detector measures the intensity of the electrons emerging from the magnetic field as a function of z. We know that • 46 of the 47 electrons of a silver atom form a spherically symmetric shell and the angular momentum of the electron outside the shell is zero, so the magnetic moment due to the or ...
... • a detector measures the intensity of the electrons emerging from the magnetic field as a function of z. We know that • 46 of the 47 electrons of a silver atom form a spherically symmetric shell and the angular momentum of the electron outside the shell is zero, so the magnetic moment due to the or ...
Solution - IISER Bhopal
... order multipole transitions (magnetic dipole, electric quadrupole, · · · ) arise mathematically, and in which cases they have to be considered? [max 6 lines]. ...
... order multipole transitions (magnetic dipole, electric quadrupole, · · · ) arise mathematically, and in which cases they have to be considered? [max 6 lines]. ...
Final Question
... For the following table, tell whether y varies directly with x. If it does, write a function rule for the relationship shown in the data. ...
... For the following table, tell whether y varies directly with x. If it does, write a function rule for the relationship shown in the data. ...
Sample pages 1 PDF
... wavelength λ ≈ 4 × 10−13 m. In other words, a neutron moving with that speed can be considered a de Broglie wave with a wavelength of 4 × 10−13 m. Similar wavelengths are characteristic of cosmic rays. Particles with a mass much larger than that of the neutron, even when moving at a much lower speed ...
... wavelength λ ≈ 4 × 10−13 m. In other words, a neutron moving with that speed can be considered a de Broglie wave with a wavelength of 4 × 10−13 m. Similar wavelengths are characteristic of cosmic rays. Particles with a mass much larger than that of the neutron, even when moving at a much lower speed ...
More on the Standard Model
... Only one fermion can be an any state, which explains most of chemistry…the electrons fill up the energy levels with only one per state. This is the Pauli exclusion principle. But can’t I put two electrons per state? Yes, but their spins are in different directions, so they are not really in the same ...
... Only one fermion can be an any state, which explains most of chemistry…the electrons fill up the energy levels with only one per state. This is the Pauli exclusion principle. But can’t I put two electrons per state? Yes, but their spins are in different directions, so they are not really in the same ...
History of The Atom2014 (1)
... • Rutherford’s nuclear model does not obey classical laws of physics….as electrons orbit around the nucleus they continuously lose energy and therefore they should spiral into the nucleus! Atoms can’t exist!?!? ...
... • Rutherford’s nuclear model does not obey classical laws of physics….as electrons orbit around the nucleus they continuously lose energy and therefore they should spiral into the nucleus! Atoms can’t exist!?!? ...
Pearson Physics Level 30 Unit VIII Atomic Physics: Chapter 17
... (e) The short tracks are likely secondary, weak collisions between the positive charge and other hydrogen nuclei in the bubble chamber. Extension 22. Pauli’s exclusion principle deals with the odd property of spin of electrons and many other subatomic particles. An electron can have only two possibl ...
... (e) The short tracks are likely secondary, weak collisions between the positive charge and other hydrogen nuclei in the bubble chamber. Extension 22. Pauli’s exclusion principle deals with the odd property of spin of electrons and many other subatomic particles. An electron can have only two possibl ...
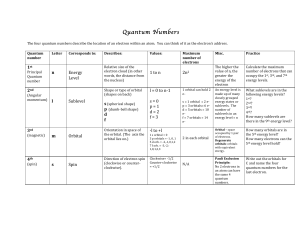
Quantum Numbers Handout File
... energy!states!or! sublevels.!!The! number!of! sublevels!in!an! energy!level!=!n! ...
... energy!states!or! sublevels.!!The! number!of! sublevels!in!an! energy!level!=!n! ...
No Slide Title
... 2 = 3 SV /V For a given voltage deviation and noise spectral density, increasing the VCO tuning parameter increases the phase error during a pulse. ...
... 2 = 3 SV /V For a given voltage deviation and noise spectral density, increasing the VCO tuning parameter increases the phase error during a pulse. ...
Linear Transformations and Matrix Algebra
... Answer each true/false question in the chapter 1 supplemental section on page 102. It is not necessary to supply justifications but if you want your logic checked then feel free to provide a justification. ...
... Answer each true/false question in the chapter 1 supplemental section on page 102. It is not necessary to supply justifications but if you want your logic checked then feel free to provide a justification. ...