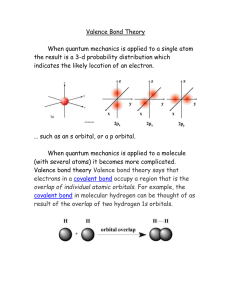
Valence Bond Theory
... electrons in a covalent bond occupy a region that is the overlap of individual atomic orbitals. For example, the covalent bond in molecular hydrogen can be thought of as result of the overlap of two hydrogen 1s orbitals. ...
... electrons in a covalent bond occupy a region that is the overlap of individual atomic orbitals. For example, the covalent bond in molecular hydrogen can be thought of as result of the overlap of two hydrogen 1s orbitals. ...
3 section 4.2
... The most likely location of an electron is described by a wave of probability. This type of wave is actually a set pattern that forms a 3-D shape within the space of the atom. This wave pattern does not overlap itself and is known as a standing wave. http://www.youtube.com/watch?v=-gr7KmTOrx0 http: ...
... The most likely location of an electron is described by a wave of probability. This type of wave is actually a set pattern that forms a 3-D shape within the space of the atom. This wave pattern does not overlap itself and is known as a standing wave. http://www.youtube.com/watch?v=-gr7KmTOrx0 http: ...
LECTURE 6
... A pretty good way to determine the electronic con gurations of the elements is to imagine adding one electron at a time to the energy levels of an atom. Each electron is added in accordance with the Pauli exclusion principle and Hund's rule. Several factors determine the energy of an electron in an ...
... A pretty good way to determine the electronic con gurations of the elements is to imagine adding one electron at a time to the energy levels of an atom. Each electron is added in accordance with the Pauli exclusion principle and Hund's rule. Several factors determine the energy of an electron in an ...
Lecture notes in Solid State 3 Eytan Grosfeld
... bound to the nucleus and form the (immobile, positively charged) metallic ion, while the (mobile, negatively charged) valence electrons are allowed to wander far away from the parent atom. In this context they are called the conduction electrons. 3. Electrons are almost free (no forces act between e ...
... bound to the nucleus and form the (immobile, positively charged) metallic ion, while the (mobile, negatively charged) valence electrons are allowed to wander far away from the parent atom. In this context they are called the conduction electrons. 3. Electrons are almost free (no forces act between e ...
ET3034TUx -‐ 2.2.1 – Band Gap I: Electrons in Atoms
... In its nucleus it has two positrons and two neutrons. Since neutrons do not have a charge, the charge of the nucleus is determined by the two positively charged positrons. Around ...
... In its nucleus it has two positrons and two neutrons. Since neutrons do not have a charge, the charge of the nucleus is determined by the two positively charged positrons. Around ...
5. Quantum mechanics of chemical binding
... • use the Aufbau principle to occupy the orbitals and get the configurations; • obtain the states. ...
... • use the Aufbau principle to occupy the orbitals and get the configurations; • obtain the states. ...
Electronic Structure of Atoms
... • It cannot explain the spectra of atoms other than hydrogen. • Electrons do not move about the nucleus in circular orbits. However, the model introduces two important ideas: • The energy of an electron is quantized: electrons exist only in certain energy levels described by quantum numbers. • Energ ...
... • It cannot explain the spectra of atoms other than hydrogen. • Electrons do not move about the nucleus in circular orbits. However, the model introduces two important ideas: • The energy of an electron is quantized: electrons exist only in certain energy levels described by quantum numbers. • Energ ...
Document
... The nuclei of all atoms of a particular element must contain the same number of protons. They may contain varying numbers of neutrons. Isotopes of an element have the same Z but differing N and A values. Example: 11 12 13 14 ...
... The nuclei of all atoms of a particular element must contain the same number of protons. They may contain varying numbers of neutrons. Isotopes of an element have the same Z but differing N and A values. Example: 11 12 13 14 ...
Name: Midterm Review (Part II) Fill in the blanks (Chapter 6.1 – 6.3
... When atom is the ground state, what must happen for the atom to be in an excited state? Your notes What must happen for this atom to return to ground state?p. 142 Would an electron have to absorb or release energy to jump from the second energy level to the third energy level?p. 142-143 How light is ...
... When atom is the ground state, what must happen for the atom to be in an excited state? Your notes What must happen for this atom to return to ground state?p. 142 Would an electron have to absorb or release energy to jump from the second energy level to the third energy level?p. 142-143 How light is ...
Ch. 5 Notes: Electrons in Atoms Big Idea: The Atoms of each
... a. Quantum- the minimum amount of energy that can be gained or lost by an atom. 3. Energy of a Quantum a. E quantum = hv ...
... a. Quantum- the minimum amount of energy that can be gained or lost by an atom. 3. Energy of a Quantum a. E quantum = hv ...
B - Piazza
... where A and B are positive constants. Because of the complicated shielding effects of the various electron shells, n and m are not equal to 1. One example is the Lennard-Jones potential in which n = 12 and m = 6. ...
... where A and B are positive constants. Because of the complicated shielding effects of the various electron shells, n and m are not equal to 1. One example is the Lennard-Jones potential in which n = 12 and m = 6. ...
P301_2009_week9
... which says that you cannot know precisely more than one component of the angular momentum. Comment on the connection between this result and the relation between |Lz| and (|L|2)1/2. •I am not going to lie, I cannot quite figure out what this question is asking for. (I think that this was true for ma ...
... which says that you cannot know precisely more than one component of the angular momentum. Comment on the connection between this result and the relation between |Lz| and (|L|2)1/2. •I am not going to lie, I cannot quite figure out what this question is asking for. (I think that this was true for ma ...
The Periodic Table - Mrs Molchany`s Webpage
... outermost electron of an atom. Li(g) → Li+(g)+ e- ionization energy 8.64 x 10-19 J/atom HIGH ionization energy means the atom hold onto the electron tightly. LOW ionization energy means the atom holds onto the electron loosely. Since an atom is very small, scientists use a larger unit of measure cal ...
... outermost electron of an atom. Li(g) → Li+(g)+ e- ionization energy 8.64 x 10-19 J/atom HIGH ionization energy means the atom hold onto the electron tightly. LOW ionization energy means the atom holds onto the electron loosely. Since an atom is very small, scientists use a larger unit of measure cal ...
Module 2 ATOMIC STRUCTURE
... classical mechanics a particle can possess any amount of energy between zero and infinity. Also, the position and velocity of the particle can be determined simultaneously. Newton’s law of motion and Maxwell’s electromagnetic wave theory successfully explained most of the phenomena known then. But w ...
... classical mechanics a particle can possess any amount of energy between zero and infinity. Also, the position and velocity of the particle can be determined simultaneously. Newton’s law of motion and Maxwell’s electromagnetic wave theory successfully explained most of the phenomena known then. But w ...
F1 In the Bohr model, the quantum number n gives the orbital
... Looking at Figure 6, points in the x–y plane correspond to values of θ = π 2 (i.e. 90°). (i) Points on the x-axis have values of φ = 0. The point (2,10,10) is two units away from the origin; so the Cartesian coordinates (2,10,10) have the (r,1θ,1φ) equivalent of (2,1π/2,10). (ii) The point (1,11,10) ...
... Looking at Figure 6, points in the x–y plane correspond to values of θ = π 2 (i.e. 90°). (i) Points on the x-axis have values of φ = 0. The point (2,10,10) is two units away from the origin; so the Cartesian coordinates (2,10,10) have the (r,1θ,1φ) equivalent of (2,1π/2,10). (ii) The point (1,11,10) ...
No Slide Title
... Effective nuclear charge (Zeff) is the “positive charge” felt by an electron. Zeff = Z - s ...
... Effective nuclear charge (Zeff) is the “positive charge” felt by an electron. Zeff = Z - s ...
Development of Bohr model due to atomic emission spectra of some
... philosophical beginnings of Democritus over Dalton’s theorems to Rutherford’s orbit theory, more and more improvements were added to the model. However, all atomic models until this point saw the atom as a particle-alike object. In 1913 Niels Bohr introduced a new approach to the structural composit ...
... philosophical beginnings of Democritus over Dalton’s theorems to Rutherford’s orbit theory, more and more improvements were added to the model. However, all atomic models until this point saw the atom as a particle-alike object. In 1913 Niels Bohr introduced a new approach to the structural composit ...
notes-2 - KSU Physics
... We next generalize the concept to 2D and 1D systems. For the quantum slabs, assuming that Lz is much smaller in dimension so in the z-direction we will assume that the wavefunction vanishes at z=0 and Lz, while the periodic boundary conditions are applied in the other two directions. Clearly the en ...
... We next generalize the concept to 2D and 1D systems. For the quantum slabs, assuming that Lz is much smaller in dimension so in the z-direction we will assume that the wavefunction vanishes at z=0 and Lz, while the periodic boundary conditions are applied in the other two directions. Clearly the en ...
The Pauli-Exclusion Principle Indistinguishability
... just the hydrogen orbitals (one for each electron). The overall solution would just be the anti-symmetric combination of hydrogen orbitals. Thus, we can obtain an approximate solution and learn a lot about atomic structure if we just neglect electron repulsion compared to the nuclear attraction. The ...
... just the hydrogen orbitals (one for each electron). The overall solution would just be the anti-symmetric combination of hydrogen orbitals. Thus, we can obtain an approximate solution and learn a lot about atomic structure if we just neglect electron repulsion compared to the nuclear attraction. The ...
Exam 3 Review - Iowa State University
... 9. Which has the largest 2nd Ionization energy between K and Ca? a. K b. Ca c. Both K and Ca have the same second Ionization energy d. It’s impossible to tell 10. In terms of electronegativity, determine whether the following compounds contain nonpolar covalent, polar covalent, or ionic bonds. a. I— ...
... 9. Which has the largest 2nd Ionization energy between K and Ca? a. K b. Ca c. Both K and Ca have the same second Ionization energy d. It’s impossible to tell 10. In terms of electronegativity, determine whether the following compounds contain nonpolar covalent, polar covalent, or ionic bonds. a. I— ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.