Lecture 1 Atomic Structure
... • The probability of finding an electron at a given location is proportional to y2 at that point. ...
... • The probability of finding an electron at a given location is proportional to y2 at that point. ...
L 35 Modern Physics [1] Modern Physics
... and the Bohr Atom • Niels Bohr, a Danish physicist, used the quantum concept to explain the nature of the atom. • Recall that the orbiting electrons, according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a ...
... and the Bohr Atom • Niels Bohr, a Danish physicist, used the quantum concept to explain the nature of the atom. • Recall that the orbiting electrons, according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a ...
Modern Physics 342
... It was believed that different atoms in the ground states have all their electrons dropped down in the 1s state. This means they all must have the same physical properties. This is not the case, in fact. A conclusion was drawn by Pauli that states that: No two electrons in a single atom can have the ...
... It was believed that different atoms in the ground states have all their electrons dropped down in the 1s state. This means they all must have the same physical properties. This is not the case, in fact. A conclusion was drawn by Pauli that states that: No two electrons in a single atom can have the ...
Chemical Bond – a force that holds two atoms together, the bond
... Chemical Bond – a force that holds two atoms together, the bond could be between two elements that are the same element or different elements. Ionic Bond – an electrostatic force between two different atomic elements (atomic nonmetal and an atomic metal) in which the atomic nonmetal steals the avail ...
... Chemical Bond – a force that holds two atoms together, the bond could be between two elements that are the same element or different elements. Ionic Bond – an electrostatic force between two different atomic elements (atomic nonmetal and an atomic metal) in which the atomic nonmetal steals the avail ...
Ch 5 - Electrons in Atoms
... • The electrons can emit or absorb only certain amounts of energy There is one issue with this model: • Unfortunately this model’s calculations ONLY worked for Hydrogen b/c Bohr didn’t understand how electrons actually moved in an atom ...
... • The electrons can emit or absorb only certain amounts of energy There is one issue with this model: • Unfortunately this model’s calculations ONLY worked for Hydrogen b/c Bohr didn’t understand how electrons actually moved in an atom ...
... When electrons are confined to a small region of a semiconductor they form a quantum dot, and the energy and the charge on the quantum dot are quantized. It has been possible to study the transmission of electrons through a quantum dot by coupling the states in the dot to external leads via a tunnel ...
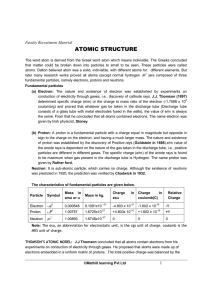
atomic structure
... (b) Proton: A proton is a fundamental particle with a charge equal in magnitude but opposite in sign to the charge on the electron, and having a much larger mass. The nature and existence of proton was established by the discovery of Positive rays (Goldstein in 1886).e/m value of the anode rays is d ...
... (b) Proton: A proton is a fundamental particle with a charge equal in magnitude but opposite in sign to the charge on the electron, and having a much larger mass. The nature and existence of proton was established by the discovery of Positive rays (Goldstein in 1886).e/m value of the anode rays is d ...
1411-Practice Exam 3 (ch6-8)
... 34. Consider the following atoms and ions; Cr, Cr3+, Bra) write electron configuration for Brb) write core configuration Cr3+ c) draw energy diagram representation for Cr d)determine the total number of unpaired electrons in Cr e) identify Cr3+ as paramagnetic or diamagnetic f) which block does Br b ...
... 34. Consider the following atoms and ions; Cr, Cr3+, Bra) write electron configuration for Brb) write core configuration Cr3+ c) draw energy diagram representation for Cr d)determine the total number of unpaired electrons in Cr e) identify Cr3+ as paramagnetic or diamagnetic f) which block does Br b ...
“Location” of Electrons in the Quantum Mechanical Model
... • Schrodinger’s wave equations reveal areas of high “electron density” – Although we don’t know for sure, we have a good idea where we can most likely find an electron ...
... • Schrodinger’s wave equations reveal areas of high “electron density” – Although we don’t know for sure, we have a good idea where we can most likely find an electron ...
Midterm Review File
... 25. If an atom has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2, how many valence electrons does it have? ...
... 25. If an atom has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2, how many valence electrons does it have? ...
Electrical Properties
... The core levels continue to remain discrete. Bands may overlap and fill in parallel over a range of energy values, as shown in the figure. As a range of energy values are allowed in a band (discrete but closely spaced), any radiative transition from these outer levels to a core levels has a br ...
... The core levels continue to remain discrete. Bands may overlap and fill in parallel over a range of energy values, as shown in the figure. As a range of energy values are allowed in a band (discrete but closely spaced), any radiative transition from these outer levels to a core levels has a br ...
Atoms and quantum phenomena
... what happens in different situations. Neither is perfect. • Louis de Broglie suggested that this same kind of dual nature must also be applicable to matter. He proposed that any particle of momentum (p) has an associated wavelength ...
... what happens in different situations. Neither is perfect. • Louis de Broglie suggested that this same kind of dual nature must also be applicable to matter. He proposed that any particle of momentum (p) has an associated wavelength ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.

![L 35 Modern Physics [1] - University of Iowa Physics](http://s1.studyres.com/store/data/000679677_1-b925cf8c8f031b0f2b0c09a806312d20-300x300.png)
![L 35 Modern Physics [1] Modern Physics](http://s1.studyres.com/store/data/001558975_1-84d6e03bc786b63795533f59711ce2f4-300x300.png)