File
... The nucleus contains less than half the mass of the atom. The nucleus is small and is the densest part of the atom. The nucleus contains small positive and negative particles. The nucleus is large and occupies most of the atom’s space. ...
... The nucleus contains less than half the mass of the atom. The nucleus is small and is the densest part of the atom. The nucleus contains small positive and negative particles. The nucleus is large and occupies most of the atom’s space. ...
Chemistry -- Oxidation
... Displacement reactions: an ion (or atom) in a compound is replaced by an ion (or atom) of another element ...
... Displacement reactions: an ion (or atom) in a compound is replaced by an ion (or atom) of another element ...
Biol 1406 notes Ch 2 8thed
... o Because potential energy has been expended, the water stores less energy at the bottom of the dam than it did in the reservoir. Electrons have potential energy because of their positions relative to the nucleus. o The negatively charged electrons are attracted to the positively charged nucleus. ...
... o Because potential energy has been expended, the water stores less energy at the bottom of the dam than it did in the reservoir. Electrons have potential energy because of their positions relative to the nucleus. o The negatively charged electrons are attracted to the positively charged nucleus. ...
Assignment 8 - Duke Physics
... (These experimental curves can be obtained by putting the molecules in solution, shining light of a known initial intensity I0 and known wavelength λ through the solution, and then by measuring the light intensity I that comes out of the solution. The absorption curve is then proportional to 1 − I/ ...
... (These experimental curves can be obtained by putting the molecules in solution, shining light of a known initial intensity I0 and known wavelength λ through the solution, and then by measuring the light intensity I that comes out of the solution. The absorption curve is then proportional to 1 − I/ ...
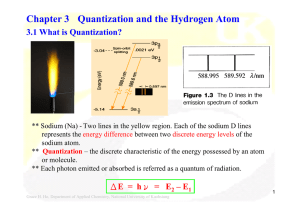
Chapter 3 Quantization and the Hydrogen Atom
... H + hν H+ + eThe ejected electron can have any amount of kinetic energy, the energy levels becomes no longer discrete but form a continuum beyond the ionization limit. ...
... H + hν H+ + eThe ejected electron can have any amount of kinetic energy, the energy levels becomes no longer discrete but form a continuum beyond the ionization limit. ...
Chemistry 199 - Oregon State chemistry
... What is a Lewis base? What is a Lewis acid? Let me start by stating that we are familiar with many bases and acids. Those we know to be bases are Lewis bases and those we know to be acids are Lewis acids. Our previous ideas of bases and acids came from Arrhenius, Bronsted, and Lowry. These ideas inv ...
... What is a Lewis base? What is a Lewis acid? Let me start by stating that we are familiar with many bases and acids. Those we know to be bases are Lewis bases and those we know to be acids are Lewis acids. Our previous ideas of bases and acids came from Arrhenius, Bronsted, and Lowry. These ideas inv ...
The Periodic Table HL Page 1 of 3 G. Galvin Name: Periodic Table
... • He switched some pairs of elements in his table so they would fit in the with the properties expected in that group • Transition metals did not have a separate block 4. Mosely: Arranged elements in order of increasing atomic number. Defn: The atomic number of an atom is the number of protons in th ...
... • He switched some pairs of elements in his table so they would fit in the with the properties expected in that group • Transition metals did not have a separate block 4. Mosely: Arranged elements in order of increasing atomic number. Defn: The atomic number of an atom is the number of protons in th ...
discrete spectra - Project PHYSNET
... electron from an initially neutral atom, leaving a system consisting of a nucleus of charge +Ze and a single electron. • ionization: the process of adding electrons to, or removing electrons from, an initially neutral atom to form a charged particle called an “ion.” By removing all the electrons, th ...
... electron from an initially neutral atom, leaving a system consisting of a nucleus of charge +Ze and a single electron. • ionization: the process of adding electrons to, or removing electrons from, an initially neutral atom to form a charged particle called an “ion.” By removing all the electrons, th ...
Chapter 2. The Chemical Context of Life
... Why are we studying chemistry? Biology has chemistry at its foundation ...
... Why are we studying chemistry? Biology has chemistry at its foundation ...
AP Chemistry Summer Study Guide
... Orbital: Regions of probability where electrons are located. Each orbital can contain up to 2 electrons Oxidation Number: A charge assigned to an atom that represents that charge it would have if it contained and ionic bond. Oxidation numbers are written as charge value, +4, -6, +2 Oxidation: Proces ...
... Orbital: Regions of probability where electrons are located. Each orbital can contain up to 2 electrons Oxidation Number: A charge assigned to an atom that represents that charge it would have if it contained and ionic bond. Oxidation numbers are written as charge value, +4, -6, +2 Oxidation: Proces ...
REVIEW OF WAVE MECHANICS
... The fact that n > l is the only effect the centrifugal potential has on the energy of the solutions is remarkable, and is only true for the 1/r Coulomb potential. In other atoms where the nucleus is partially screened by the inner electrons, the potential seen by the outer electron is not of this si ...
... The fact that n > l is the only effect the centrifugal potential has on the energy of the solutions is remarkable, and is only true for the 1/r Coulomb potential. In other atoms where the nucleus is partially screened by the inner electrons, the potential seen by the outer electron is not of this si ...
Hydrogen and the Central Force Problem
... (antielectron) attract each other with a potential related to their separation. The one dimensional analogy would be V (x1 − x2 ). It is very difficult to separate variables for this potential in the original (x1 , x2 ) coordinates but there is a classic change of variables trick which allows for a se ...
... (antielectron) attract each other with a potential related to their separation. The one dimensional analogy would be V (x1 − x2 ). It is very difficult to separate variables for this potential in the original (x1 , x2 ) coordinates but there is a classic change of variables trick which allows for a se ...
Lecture 11 - 12 - Cambridge University Press
... Today, quantum mechanics is the basis for understanding physical phenomena on the atomic and nano-meter scale. There are numerous applications of quantum mechanics in biology, chemistry and engineering. Those with significant economic impact include semiconductor transistors, lasers, quantum optics ...
... Today, quantum mechanics is the basis for understanding physical phenomena on the atomic and nano-meter scale. There are numerous applications of quantum mechanics in biology, chemistry and engineering. Those with significant economic impact include semiconductor transistors, lasers, quantum optics ...
A Binary Star as a Quantum System
... If M J ® ¥, probability is proportional to Df No f is favored ...
... If M J ® ¥, probability is proportional to Df No f is favored ...
FirstSemesterReviewHonors
... You may use the study guide on the final exam. You must provide all formulas where needed, since formulas will not be provided for you on the final. You should take at least 1 week to complete the material within the study guide. Chapter 1 1. A characteristic of a scientific theory is that it can ne ...
... You may use the study guide on the final exam. You must provide all formulas where needed, since formulas will not be provided for you on the final. You should take at least 1 week to complete the material within the study guide. Chapter 1 1. A characteristic of a scientific theory is that it can ne ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.