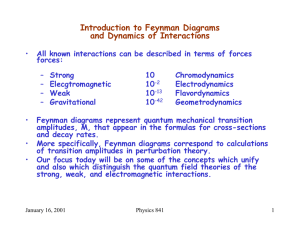
Need
... All elements with an atomic number higher than 83 are radioactive. 2. Each isotope has a specific mode and rate of decay. (see Table N) The rate of decay is called half life. Half-life is a constant that can never be changed. Half life is the measure of the time it takes exactly one half of ...
... All elements with an atomic number higher than 83 are radioactive. 2. Each isotope has a specific mode and rate of decay. (see Table N) The rate of decay is called half life. Half-life is a constant that can never be changed. Half life is the measure of the time it takes exactly one half of ...
Slide1
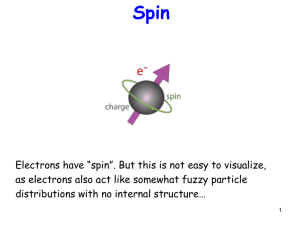
... Spin is like angular momentum Recall m can have (2l+1) values between –l and l. For spin, since only 2 ...
... Spin is like angular momentum Recall m can have (2l+1) values between –l and l. For spin, since only 2 ...
Notes
... The number of electrons ________________ by the species being oxidized must always equal the number of electrons ________________ by the species being reduced. ...
... The number of electrons ________________ by the species being oxidized must always equal the number of electrons ________________ by the species being reduced. ...
Superluminal Quantum Models of the Photon and Electron
... In 1925, Werner Heisenberg introduced matrix mechanics to describe what is observable about radiation from atoms – light frequencies and intensities. In 1926, Erwin Schrodinger in introduced wave mechanics to predict the observed energy levels of atoms based on electron wave properties. The two theo ...
... In 1925, Werner Heisenberg introduced matrix mechanics to describe what is observable about radiation from atoms – light frequencies and intensities. In 1926, Erwin Schrodinger in introduced wave mechanics to predict the observed energy levels of atoms based on electron wave properties. The two theo ...
Uncertainty Principle
... Indeterminate Newtonian physics is viewed as a deterministic system. • Initial positions allow calculation of final states • Knowledge of all past variables implies future knowledge ...
... Indeterminate Newtonian physics is viewed as a deterministic system. • Initial positions allow calculation of final states • Knowledge of all past variables implies future knowledge ...
Atoms and Periodic Table Unit Name
... 25 - These are good conductors of heat and electricity. They also have luster and a high density 27 - Metals are considered this if they can be made into wire. 29 - There are this many known quarks? 30 - The attraction that holds atoms close to each other 32 - Group of nitrogenous organic compounds ...
... 25 - These are good conductors of heat and electricity. They also have luster and a high density 27 - Metals are considered this if they can be made into wire. 29 - There are this many known quarks? 30 - The attraction that holds atoms close to each other 32 - Group of nitrogenous organic compounds ...
CHEM 400 - El Camino College
... Distinguish between heat at constant volume (qv = ΔE) and heat at constant pressure (qp = ΔH). Concept of enthalpy. Why do chemists prefer to use enthalpy rather that internal energy? Know how calorimetry can be used to determine specific heats of substances and heats of reactions. What are the adva ...
... Distinguish between heat at constant volume (qv = ΔE) and heat at constant pressure (qp = ΔH). Concept of enthalpy. Why do chemists prefer to use enthalpy rather that internal energy? Know how calorimetry can be used to determine specific heats of substances and heats of reactions. What are the adva ...
Review for second exam:
... metals, nonmetals, metalloids (semimetals); general properties and location Effective nuclear charge, Zeff; approximate value for Zeff, calculation and interpretation Zeff and Coulomb’s law Trends in atomic size (atoms in the same group, atoms in the same row); explanation for trends Definition of f ...
... metals, nonmetals, metalloids (semimetals); general properties and location Effective nuclear charge, Zeff; approximate value for Zeff, calculation and interpretation Zeff and Coulomb’s law Trends in atomic size (atoms in the same group, atoms in the same row); explanation for trends Definition of f ...
2. Essential Chemistry
... o The plus sign (+) means “react” and the arrow points towards the substance produce in the reaction o The chemical formulas on the right side of the equation are called reactants and after the arrow are called product o The numbers in front of the molecules or atoms indicate the number of individua ...
... o The plus sign (+) means “react” and the arrow points towards the substance produce in the reaction o The chemical formulas on the right side of the equation are called reactants and after the arrow are called product o The numbers in front of the molecules or atoms indicate the number of individua ...
First Semester complete review with answers
... 33. How do you determine an element’s oxidation number? Use potassium and nitrogen as examples. Oxidation number is determined y how many electrons an atom takes or gives to become an ion. K oxidation number is +1. Potassium (K) is in group 1 and has 1 valence electron. K gives up that 1 electron be ...
... 33. How do you determine an element’s oxidation number? Use potassium and nitrogen as examples. Oxidation number is determined y how many electrons an atom takes or gives to become an ion. K oxidation number is +1. Potassium (K) is in group 1 and has 1 valence electron. K gives up that 1 electron be ...
File
... of ejected electrons should increase. Electrons should be emitted whatever the frequency ν of the light, so long as E is sufficiently large I Ammeter Potentiostat ...
... of ejected electrons should increase. Electrons should be emitted whatever the frequency ν of the light, so long as E is sufficiently large I Ammeter Potentiostat ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.