Link between the hierarchy of fractional quantum Hall states and
... Link between the hierarchy of fractional quantum Hall states and Haldane’s conjecture for quantum spin chains Masaaki Nakamura Department of Physics, Tokyo Institute of Technology, Tokyo 152-8551, Japan ...
... Link between the hierarchy of fractional quantum Hall states and Haldane’s conjecture for quantum spin chains Masaaki Nakamura Department of Physics, Tokyo Institute of Technology, Tokyo 152-8551, Japan ...
Chapter 4.2 Quantum Models
... The Heisenberg uncertainty principle states that it is impossible to determine simultaneously both the position and velocity of an electron or any other particle ...
... The Heisenberg uncertainty principle states that it is impossible to determine simultaneously both the position and velocity of an electron or any other particle ...
Quarter Exam (Old Test)
... c. A compound consists of more than one phase. d. A compound can only be separated into its components by chemical means. ____ 29. What is the formula unit of sodium nitride? a. NaN b. NaN ...
... c. A compound consists of more than one phase. d. A compound can only be separated into its components by chemical means. ____ 29. What is the formula unit of sodium nitride? a. NaN b. NaN ...
Limits of classical physics II.
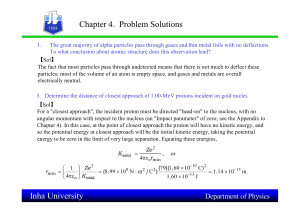
... Electrons make circular motion around the core of atom due to the centripetal force originating from the core. Something is wrong with the atom model of Rutherford: If the electron moves on a circle (circle motion with constant speed) Electrons should move as an accelerating particle acceleratin ...
... Electrons make circular motion around the core of atom due to the centripetal force originating from the core. Something is wrong with the atom model of Rutherford: If the electron moves on a circle (circle motion with constant speed) Electrons should move as an accelerating particle acceleratin ...
1411-Practice Exam 3 (ch6-8)
... Sodium and potassium have similar chemical and physical properties. This is best explained by the fact that both elements A) are active metals. B) are in Period 1 of the periodic table. C) have the same ground-state valence-electron configuration. D) have low relative atomic masses. E) have relative ...
... Sodium and potassium have similar chemical and physical properties. This is best explained by the fact that both elements A) are active metals. B) are in Period 1 of the periodic table. C) have the same ground-state valence-electron configuration. D) have low relative atomic masses. E) have relative ...
Properties of the Physical Universe
... The main properties of an atom are defined by the contents of its nucleus. In fact, the type of element an atom is defined to be is dictated by the number of protons in the nucleus. This property is called the atomic number (Z). For example, the element Hydrogen is defined as an atom that possesses ...
... The main properties of an atom are defined by the contents of its nucleus. In fact, the type of element an atom is defined to be is dictated by the number of protons in the nucleus. This property is called the atomic number (Z). For example, the element Hydrogen is defined as an atom that possesses ...
6. Quantum Mechanics II
... which is a sine wave moving in the x direction. Notice that, unlike classical waves, we are not taking the real part of this function. is, in fact, complex. In general, the wave function is complex. But the physically measurable quantities must be real. These include the probability, position, mom ...
... which is a sine wave moving in the x direction. Notice that, unlike classical waves, we are not taking the real part of this function. is, in fact, complex. In general, the wave function is complex. But the physically measurable quantities must be real. These include the probability, position, mom ...
CHM 441: QUANTUM CHEMISTRY
... electron would inevitably be after in the process. This would definitely limit our ability to measure the momentum. If we use a photon of shorter wavelength to determine the position of the electron more accurately, the disturbance of the momentum is greater and ∆px is greater according to equation ...
... electron would inevitably be after in the process. This would definitely limit our ability to measure the momentum. If we use a photon of shorter wavelength to determine the position of the electron more accurately, the disturbance of the momentum is greater and ∆px is greater according to equation ...
Atomic Structure and Periodic Trends
... Next round of research • Goal was to describe electrons in atoms • Ultimately describe for each electron: – Energy level & size of the region it occupies (n) – 3-D shape of the region it occupies (l) – Orientation of the region/orbital (ml) – Spin on the electron (ms) ...
... Next round of research • Goal was to describe electrons in atoms • Ultimately describe for each electron: – Energy level & size of the region it occupies (n) – 3-D shape of the region it occupies (l) – Orientation of the region/orbital (ml) – Spin on the electron (ms) ...
Chapter 6: Electronic Structure of Atoms
... Niels Bohr adopted Planck’s assumption and explained these phenomena: 1. Electrons in an atom can only occupy certain orbits (corresponding to certain energies). 2. Electrons in permitted orbits have specific, “allowed” energies; these energies will not be radiated from the atom. 3. Energy is only a ...
... Niels Bohr adopted Planck’s assumption and explained these phenomena: 1. Electrons in an atom can only occupy certain orbits (corresponding to certain energies). 2. Electrons in permitted orbits have specific, “allowed” energies; these energies will not be radiated from the atom. 3. Energy is only a ...
ZCT 104 Test II solution
... Balmer series corresponds to the spectral lines emitted when the electron in a hydrogen atom makes transitions from higher states to the n = 1 state II(F) Lyman series corresponds to the spectral lines emitted when the electron in a hydrogen atom makes transitions from higher states to the n = 2 sta ...
... Balmer series corresponds to the spectral lines emitted when the electron in a hydrogen atom makes transitions from higher states to the n = 1 state II(F) Lyman series corresponds to the spectral lines emitted when the electron in a hydrogen atom makes transitions from higher states to the n = 2 sta ...
Planck`s Law and Light Quantum Hypothesis.
... that is, the relation between the radiation density and the mean energy of an oscillator, and they make assumptions about the number of degrees of freedom of the ether, which appear in the above formula (the first factor on the right– hand side). This factor, however, can be derived only from classi ...
... that is, the relation between the radiation density and the mean energy of an oscillator, and they make assumptions about the number of degrees of freedom of the ether, which appear in the above formula (the first factor on the right– hand side). This factor, however, can be derived only from classi ...
5-2 Quantum Theory and the Atom
... Other electron transitions have been measured that are not visible, such as the Lyman series (ultraviolet), in which the electron drop into the n=1 orbit, and the Paschen series (infrared), in which the electrons drop onto the n=3 orbit. READING CHECK Explain why different colors of light resu ...
... Other electron transitions have been measured that are not visible, such as the Lyman series (ultraviolet), in which the electron drop into the n=1 orbit, and the Paschen series (infrared), in which the electrons drop onto the n=3 orbit. READING CHECK Explain why different colors of light resu ...
Modern Physics 342
... The Hydrogen Atom Wave Functions • The Schrödinger Equation in Spherical Coordinates The Schrödinger equation in three dimensions is ...
... The Hydrogen Atom Wave Functions • The Schrödinger Equation in Spherical Coordinates The Schrödinger equation in three dimensions is ...
No Slide Title
... L = rXp = (ix + jy + kz)X ( ipx + jpy +kpz ) L = (r ypz - rz py)i + (r z px -r xpz )j + (r xpy - rypx)k ...
... L = rXp = (ix + jy + kz)X ( ipx + jpy +kpz ) L = (r ypz - rz py)i + (r z px -r xpz )j + (r xpy - rypx)k ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).