学术报告
... energy, the fidelity susceptibility shows distinct scaling and singular behaviours around the critical point. Secondly, I would like to introduce the relation between the fidelity susceptibility and quantum adiabatic theorem. For a d-dimensional quantum many-body system, we show that the duration ti ...
... energy, the fidelity susceptibility shows distinct scaling and singular behaviours around the critical point. Secondly, I would like to introduce the relation between the fidelity susceptibility and quantum adiabatic theorem. For a d-dimensional quantum many-body system, we show that the duration ti ...
Prerequisite Knowledge for Chemistry
... All atoms of one element will have the same number of protons. If you change the number of protons then the element changes. For instance, all carbon atoms have 6 protons. If a proton is added to a carbon atom, the atom would become nitrogen. All atoms with 7 protons are nitrogen. ...
... All atoms of one element will have the same number of protons. If you change the number of protons then the element changes. For instance, all carbon atoms have 6 protons. If a proton is added to a carbon atom, the atom would become nitrogen. All atoms with 7 protons are nitrogen. ...
Lecture 6: The Fractional Quantum Hall Effect Fractional quantum
... Landau levels, as deduced from their position on the B axis (v is the Landau level filling factor). 2. They are associated with a quantum number f = v = p/q, as determined from the concomitant quantization of the Hall resistance. 3. They are sensitive to disorder. Low-mobility samples do not show a ...
... Landau levels, as deduced from their position on the B axis (v is the Landau level filling factor). 2. They are associated with a quantum number f = v = p/q, as determined from the concomitant quantization of the Hall resistance. 3. They are sensitive to disorder. Low-mobility samples do not show a ...
PDF
... Privacy setting: h1i hTopici h81Q05i h35Q40i † This text is available under the Creative Commons Attribution/Share-Alike License 3.0. You can reuse this document or portions thereof only if you do so under terms that are compatible with the CC-BY-SA license. 1 This is in fact a little imprecise sinc ...
... Privacy setting: h1i hTopici h81Q05i h35Q40i † This text is available under the Creative Commons Attribution/Share-Alike License 3.0. You can reuse this document or portions thereof only if you do so under terms that are compatible with the CC-BY-SA license. 1 This is in fact a little imprecise sinc ...
AC Stark Effect
... Shift of atomic levels Mixing of atomic levels Splitting of atomic levels (another pretty but irrelevant picture) ...
... Shift of atomic levels Mixing of atomic levels Splitting of atomic levels (another pretty but irrelevant picture) ...
Gerard `t Hooft
... How does God produce random numbers ? Could these random numbers be actually created by “ordinary” physical processes at the Planck scale? ...
... How does God produce random numbers ? Could these random numbers be actually created by “ordinary” physical processes at the Planck scale? ...
2. Semiconductor Physics 2.1 Basic Band Theory
... This is an extremely important formula, that is easily generalized for most everything. The number (or density) of something is given by the density of available places times the probability of occupation. This applies to the number of people found in a given church or stadium, the number of photons ...
... This is an extremely important formula, that is easily generalized for most everything. The number (or density) of something is given by the density of available places times the probability of occupation. This applies to the number of people found in a given church or stadium, the number of photons ...
Lecture 1
... Generally, half-integer values are also allowed (but not for orbital angular moment). Elementary particles carry intrinsic angular momentum S in addition to L. Spin of elementary particles has nothing to do with rotation, does not depend on ...
... Generally, half-integer values are also allowed (but not for orbital angular moment). Elementary particles carry intrinsic angular momentum S in addition to L. Spin of elementary particles has nothing to do with rotation, does not depend on ...
L14alternative - Particle Physics and Particle Astrophysics
... Introduction to Quantum Mechanics Quantum indeterminacy If you bombard a hydrogen atom with photons of high enough energy to promote the electron from E1 to E3 then sometimes it will do this and other times it wont !!! The same occurs for an electron in an excited state that can either drop down on ...
... Introduction to Quantum Mechanics Quantum indeterminacy If you bombard a hydrogen atom with photons of high enough energy to promote the electron from E1 to E3 then sometimes it will do this and other times it wont !!! The same occurs for an electron in an excited state that can either drop down on ...
Is Anything Real? Have Physicists Lost Their Grip on Reality?
... Neptune: Problem and Solution In the 19th century, there were problems in analyzing the orbit of Uranus. Either Newton's mechanics were bad, or there was another planet out there. Newton's theory predicted a planet at a specific location. When we looked, it was there! It is hard to say such a theor ...
... Neptune: Problem and Solution In the 19th century, there were problems in analyzing the orbit of Uranus. Either Newton's mechanics were bad, or there was another planet out there. Newton's theory predicted a planet at a specific location. When we looked, it was there! It is hard to say such a theor ...
Worksheet 1 Answer Key from 2010
... Light is neither a wave nor a particle but rather something different which exhibits properties of both waves and particles, sometimes called a "waveicle." 2. What equation corresponds to the wave nature of light? Diagram the equation as part of your response. Note: diagramming an equation involves ...
... Light is neither a wave nor a particle but rather something different which exhibits properties of both waves and particles, sometimes called a "waveicle." 2. What equation corresponds to the wave nature of light? Diagram the equation as part of your response. Note: diagramming an equation involves ...
Lectures 6-7
... For four of the d orbitals, both of these nodes are planes, giving a ‘petal-shaped’ orbital. For the fifth d orbital (_____),the nodes look more like a pair of inverted cones. This gives an orbital that looks a bit like a p orbital with a doughnut around it. (Note the phases, though; they are differ ...
... For four of the d orbitals, both of these nodes are planes, giving a ‘petal-shaped’ orbital. For the fifth d orbital (_____),the nodes look more like a pair of inverted cones. This gives an orbital that looks a bit like a p orbital with a doughnut around it. (Note the phases, though; they are differ ...
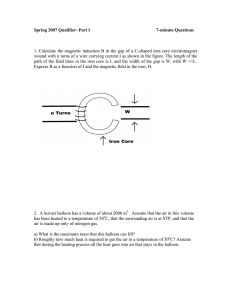
Spring 2007 Qualifier- Part I 7-minute Questions
... approximately (A) counts per second in your detector. The room background rate alone is approximately (B) counts per second. If you have a fixed total time available, what is the optimal division between time counting the source and time counting the background to minimize the statistical uncertaint ...
... approximately (A) counts per second in your detector. The room background rate alone is approximately (B) counts per second. If you have a fixed total time available, what is the optimal division between time counting the source and time counting the background to minimize the statistical uncertaint ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).