Problem Set 11 Solutions - Illinois State Chemistry
... For Si, placing two electrons in the 3p set of orbitals leads to two unpaired electrons. Thus, the total spin of the two unpaired electrons is S=1, and therefore the multiplicity is 2S+1 = 2·1+1 = 3. Therefore, the multiplicity of the ground state of Si is a triplet. For P, placing three electrons i ...
... For Si, placing two electrons in the 3p set of orbitals leads to two unpaired electrons. Thus, the total spin of the two unpaired electrons is S=1, and therefore the multiplicity is 2S+1 = 2·1+1 = 3. Therefore, the multiplicity of the ground state of Si is a triplet. For P, placing three electrons i ...
PERIODIC TABLE
... Aluminum is a lightweight metal (density = 2.70 g/cm3). What is its density in kg/m3? ...
... Aluminum is a lightweight metal (density = 2.70 g/cm3). What is its density in kg/m3? ...
UNIT 1 WORKSHEET 1. Name three methods for the separation of
... 7.69 g Z. The second sample was 35.9% A and 64.1% Z. It was observed that 0.718 g A reacted with Z to form 2.00 g of the third sample. Show that these data illustrate the law of definite composition. ...
... 7.69 g Z. The second sample was 35.9% A and 64.1% Z. It was observed that 0.718 g A reacted with Z to form 2.00 g of the third sample. Show that these data illustrate the law of definite composition. ...
Syllabus
... The most important course objectives are: 1. Student should understand properly the concepts, principles of quantum mechanics, with emphasize on the significance and interpretation of the wave function, of the uncertainty principle, of time dependent and time independent phenomena. 2. Student should ...
... The most important course objectives are: 1. Student should understand properly the concepts, principles of quantum mechanics, with emphasize on the significance and interpretation of the wave function, of the uncertainty principle, of time dependent and time independent phenomena. 2. Student should ...
2 - Castle High School
... CaCl2, and that has a mass of 400 g? • a. 15% c. 24% • b. 1.35% d. 6.7% ...
... CaCl2, and that has a mass of 400 g? • a. 15% c. 24% • b. 1.35% d. 6.7% ...
Chapter 9 Quantum Mechanics
... In the previous chapters, we have learned fundamental laws of mechanics, fluid dynamics, vibrations and waves, special relativity, surface phenomena of liquid and Optics that are parts of classical physics theories. In the above courses, the physical quantities used are continuous, such as momentum, ...
... In the previous chapters, we have learned fundamental laws of mechanics, fluid dynamics, vibrations and waves, special relativity, surface phenomena of liquid and Optics that are parts of classical physics theories. In the above courses, the physical quantities used are continuous, such as momentum, ...
PDF
... theorem is use to expand in a power series an Hermitian operator which depends on a parameter, the Planck constant, and according to the perturbation, energy associated with the interaction between dipoles is obtained, which is the potential form of the Van der Waals forces, or energy associated wit ...
... theorem is use to expand in a power series an Hermitian operator which depends on a parameter, the Planck constant, and according to the perturbation, energy associated with the interaction between dipoles is obtained, which is the potential form of the Van der Waals forces, or energy associated wit ...
chem100chapter5 - Imperial Valley College Faculty Websites
... mass units” was devised to express the masses of elements using simple ...
... mass units” was devised to express the masses of elements using simple ...
穨 Ams1a
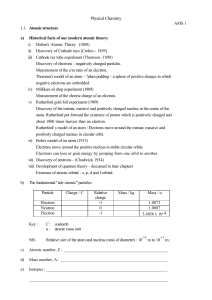
... Measurement of the electric charge of an electron. v) Rutherford gold foil experiment (1909) Discovery of the minute, massive and positively charged nucleus at the centre of the atom. Rutherford put forward the existence of proton which is positively charged and about 1800 times heavier than an elec ...
... Measurement of the electric charge of an electron. v) Rutherford gold foil experiment (1909) Discovery of the minute, massive and positively charged nucleus at the centre of the atom. Rutherford put forward the existence of proton which is positively charged and about 1800 times heavier than an elec ...
Electrons in Atoms
... 6. Which of the following best describes the Heisenberg uncertainty principle? a. Light behaves like a particle and like a wave. b. The shorter the wavelength, the higher the frequency. c. It is impossible to know both the velocity and the position of a particle at the same time. d. You cannot measu ...
... 6. Which of the following best describes the Heisenberg uncertainty principle? a. Light behaves like a particle and like a wave. b. The shorter the wavelength, the higher the frequency. c. It is impossible to know both the velocity and the position of a particle at the same time. d. You cannot measu ...
Quantum Mechanics
... The Bohr model gives us a basic conceptual model of electrons orbits and energies. The precise details of spectra and charge distribution must be left to quantum mechanical calculations, as with the Schrödinger equation. ...
... The Bohr model gives us a basic conceptual model of electrons orbits and energies. The precise details of spectra and charge distribution must be left to quantum mechanical calculations, as with the Schrödinger equation. ...
1st Semester Chem Final Exam Study Guide 2012-2013
... Be able to identify an element’s period and group. 8a. The period and group for Magnesium is ____________________. b. The electron configuration of a certain element ends in 4p4. The period and group for this element is: _______________________________ Be able to identify any element as a metal, no ...
... Be able to identify an element’s period and group. 8a. The period and group for Magnesium is ____________________. b. The electron configuration of a certain element ends in 4p4. The period and group for this element is: _______________________________ Be able to identify any element as a metal, no ...
Final Exam Review Answers
... • An atom of an element with atomic number 48 and mass number 120 contains • a. 48 protons, 48 electrons, and 72 neutrons. • b. 72 protons, 48 electrons, and 48 neutrons. • c. 120 protons, 48 electrons, and 72 neutrons. • d. 72 protons, 72 electrons, and 48 neutrons. ...
... • An atom of an element with atomic number 48 and mass number 120 contains • a. 48 protons, 48 electrons, and 72 neutrons. • b. 72 protons, 48 electrons, and 48 neutrons. • c. 120 protons, 48 electrons, and 72 neutrons. • d. 72 protons, 72 electrons, and 48 neutrons. ...
Part 2: Quantum theory of light
... of the observed spectrum could be exactly predicted if the energies emitted or absorbed by each oscillator were restricted to integral values of hn, where n is the frequency and h is a constant 6.626E-34 J s which we now know as Planck's Constant. The allowable energies of each oscillator are quanti ...
... of the observed spectrum could be exactly predicted if the energies emitted or absorbed by each oscillator were restricted to integral values of hn, where n is the frequency and h is a constant 6.626E-34 J s which we now know as Planck's Constant. The allowable energies of each oscillator are quanti ...
Spontaneous emission of an excited two
... spectra, hence more fictitious oscillators are needed to simulate them. Moreover, in the case of the rotating-wave approximation, the total quanta of the fictitious oscillators is either one or zero, while the counter-rotating wave interaction puts no limitation on the quanta numbers. Both of these wi ...
... spectra, hence more fictitious oscillators are needed to simulate them. Moreover, in the case of the rotating-wave approximation, the total quanta of the fictitious oscillators is either one or zero, while the counter-rotating wave interaction puts no limitation on the quanta numbers. Both of these wi ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).
![L 35 Modern Physics [1] Modern Physics](http://s1.studyres.com/store/data/003926344_1-b779c05b753c6dc3972377c21f9bdcd3-300x300.png)