Chemistry: Matter and Change
... • The model doesn’t explain how the electrons were arranged around the nucleus. • The model doesn’t explain why negatively charged electrons aren’t pulled into the positively charged nucleus. ...
... • The model doesn’t explain how the electrons were arranged around the nucleus. • The model doesn’t explain why negatively charged electrons aren’t pulled into the positively charged nucleus. ...
Document
... The Stern-Gerlach Experiment a) The experimental arrangement for the Stern-Gerlach experiment: the magnet provides an inhomogeneous field. b) The classically expected result. c) The observed outcome using silver atoms. ...
... The Stern-Gerlach Experiment a) The experimental arrangement for the Stern-Gerlach experiment: the magnet provides an inhomogeneous field. b) The classically expected result. c) The observed outcome using silver atoms. ...
G040162-00 - DCC
... Thermal Operator from Quantum Control Theory", by J. A. Sidles. 3) Work to establish the formal equivalence (or alternatively, the inequivalence) of the above formalisms to operator-based and field-theoretic quantum descriptions of test mass observation. ...
... Thermal Operator from Quantum Control Theory", by J. A. Sidles. 3) Work to establish the formal equivalence (or alternatively, the inequivalence) of the above formalisms to operator-based and field-theoretic quantum descriptions of test mass observation. ...
Chapter 5 Electrons in Atoms
... of atoms. Three rules tell us how: 1) Aufbau principle - electrons enter the lowest energy first. • This causes difficulties because of the overlap of orbitals of different energies – follow the diagram! 2) Pauli Exclusion Principle - at most 2 electrons per orbital - different spins ...
... of atoms. Three rules tell us how: 1) Aufbau principle - electrons enter the lowest energy first. • This causes difficulties because of the overlap of orbitals of different energies – follow the diagram! 2) Pauli Exclusion Principle - at most 2 electrons per orbital - different spins ...
Development of Bohr model due to atomic emission spectra of some
... more and more improvements were added to the model. However, all atomic models until this point saw the atom as a particle-alike object. In 1913 Niels Bohr introduced a new approach to the structural composition of an atom, in which he assumed that the electrons revolve around the nucleus on distinc ...
... more and more improvements were added to the model. However, all atomic models until this point saw the atom as a particle-alike object. In 1913 Niels Bohr introduced a new approach to the structural composition of an atom, in which he assumed that the electrons revolve around the nucleus on distinc ...
NYS Regents Chemistry June 21, 2002
... a. State the effect on the number of moles of N2(g) if the temperature of the system is increased. b. State the effect on the number of moles of H2(g) if the pressure on the system is increased. c. State the effect on the number of moles of NH3(g) if a catalyst is introduced into the reaction system ...
... a. State the effect on the number of moles of N2(g) if the temperature of the system is increased. b. State the effect on the number of moles of H2(g) if the pressure on the system is increased. c. State the effect on the number of moles of NH3(g) if a catalyst is introduced into the reaction system ...
2015 AP Chemistry Summer Assignment
... 44. Elixirs such as Alka-Seltzer use the reaction of sodium bicarbonate with citric acid in aqueous solution to produce a fizz: 3NaHCO3(aq) + C6H8O7(aq) → 3CO2(g) + 3H2O(l) + Na3C6H5O7(aq) a) What mass of C6H8O7 should be used for every 1.0 x 102 mg NaHCO3? b) What mass of CO2(g) could be produced f ...
... 44. Elixirs such as Alka-Seltzer use the reaction of sodium bicarbonate with citric acid in aqueous solution to produce a fizz: 3NaHCO3(aq) + C6H8O7(aq) → 3CO2(g) + 3H2O(l) + Na3C6H5O7(aq) a) What mass of C6H8O7 should be used for every 1.0 x 102 mg NaHCO3? b) What mass of CO2(g) could be produced f ...
ELECTRON I: Free electron model
... According to the Pauli exclusion principle, only two electrons (spin up and spin down) can occupy one state with the same energy, a state labled by quantum number n can accommodate two electrons. The Fermi energy, defined as the energy of the topmost filled state (relative to the energy of the grou ...
... According to the Pauli exclusion principle, only two electrons (spin up and spin down) can occupy one state with the same energy, a state labled by quantum number n can accommodate two electrons. The Fermi energy, defined as the energy of the topmost filled state (relative to the energy of the grou ...
3 Radiation processes 3.1 Atomic and molecular structure
... where re = e2 /me c2 = 2.8 · 10−13 cm is the classical electron radius. In the Coulomb field, the average kinetic energy is equal to the binding energy so that the electron velocity at the i-th level could be estimated as v = αc/i. For hydrogen-like ions, IZ = Z 2 IH , aZ = aH /Z. In all atoms, the ...
... where re = e2 /me c2 = 2.8 · 10−13 cm is the classical electron radius. In the Coulomb field, the average kinetic energy is equal to the binding energy so that the electron velocity at the i-th level could be estimated as v = αc/i. For hydrogen-like ions, IZ = Z 2 IH , aZ = aH /Z. In all atoms, the ...
PPT
... – The particles don't have to be detected in opposite directions, the conservation laws only hold on the average. (Bohr thought this at one time, but it's completely wrong experimentally.) – The particles are always found in opposite directions, because there is some hidden variable which allows the ...
... – The particles don't have to be detected in opposite directions, the conservation laws only hold on the average. (Bohr thought this at one time, but it's completely wrong experimentally.) – The particles are always found in opposite directions, because there is some hidden variable which allows the ...
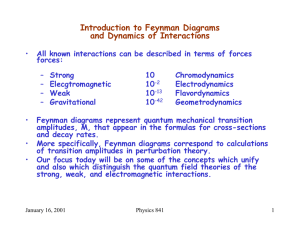
Introduction to Feynman Diagrams and Dynamics of Interactions
... perturbation theory in nonrelativistic quantum mechanics, we have second order perturbation theory in quantum field theories. ...
... perturbation theory in nonrelativistic quantum mechanics, we have second order perturbation theory in quantum field theories. ...
4.2 Discovering Parts of the Atom
... • Rutherford knew the mass of a proton, but could not account for the total mass of an atom. • Rutherford’s theory was later confirmed when the existence of the neutron—a neutral atomic particle with a mass similar to a proton but without a charge—was proved. ...
... • Rutherford knew the mass of a proton, but could not account for the total mass of an atom. • Rutherford’s theory was later confirmed when the existence of the neutron—a neutral atomic particle with a mass similar to a proton but without a charge—was proved. ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).