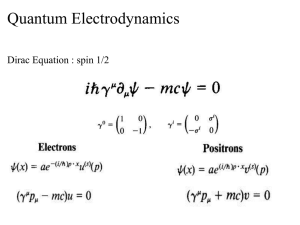
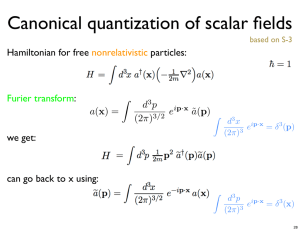
数学与系统科学研究院学术报告
... Decoherence, which is caused due to the interaction of a quantum system with its environment plagues all quantum systems and leads to the loss of quantum properties that are vital for quantum computation and quantum information processing. In this work we propose a novel strategy using techniques fr ...
... Decoherence, which is caused due to the interaction of a quantum system with its environment plagues all quantum systems and leads to the loss of quantum properties that are vital for quantum computation and quantum information processing. In this work we propose a novel strategy using techniques fr ...
Atoms, Molecules, and Ions
... All isotopes are atoms of the same element, oxygen, with the same atomic number (Z = 8), 8 protons in the nucleus and 8 electrons. Elements with similar electron arrangements have similar chemical properties (Section 2.5). Since the 3 isotopes all have 8 electrons, we expect their electron arrangeme ...
... All isotopes are atoms of the same element, oxygen, with the same atomic number (Z = 8), 8 protons in the nucleus and 8 electrons. Elements with similar electron arrangements have similar chemical properties (Section 2.5). Since the 3 isotopes all have 8 electrons, we expect their electron arrangeme ...
Teacher quality grant - Gulf Coast State College
... An atom that loses one or more electrons becomes positively charged, while an atom that acquires electrons becomes negatively charged. This transfer of electrons is driven by the fact that atoms with full outer electron shells are more stable. Donated electron ...
... An atom that loses one or more electrons becomes positively charged, while an atom that acquires electrons becomes negatively charged. This transfer of electrons is driven by the fact that atoms with full outer electron shells are more stable. Donated electron ...
Atomic Structure
... speeds. Classical mechanics studies large particles going at relatively slow speeds. Since electrons are small particles going at high speeds the electron (and thus chemistry) can only be understood through the use of quantum mechanics. ...
... speeds. Classical mechanics studies large particles going at relatively slow speeds. Since electrons are small particles going at high speeds the electron (and thus chemistry) can only be understood through the use of quantum mechanics. ...
Lesson 1 - Working With Chemicals
... o A nucleus – a central region that is positively charged and contains most of the mass - protons are heavy positive particles within the nucleus o Electrons – particles with a negative charge and are very light (compared to protons). - Electrons circle around the nucleus o Empty space surrounding t ...
... o A nucleus – a central region that is positively charged and contains most of the mass - protons are heavy positive particles within the nucleus o Electrons – particles with a negative charge and are very light (compared to protons). - Electrons circle around the nucleus o Empty space surrounding t ...
CHAPTER 3 Observation of X Rays Röntgen`s X
... Planck made two modifications to the classical theory: The oscillators (of electromagnetic origin) can only have certain discrete energies, En = nhν, where n is an integer, ν is the frequency, and h is called Planck’s constant: h = 6.6261 × 10−34 J·s. ...
... Planck made two modifications to the classical theory: The oscillators (of electromagnetic origin) can only have certain discrete energies, En = nhν, where n is an integer, ν is the frequency, and h is called Planck’s constant: h = 6.6261 × 10−34 J·s. ...
Ch 4 PPT - mvhs
... different elements is a direct consequence of wave-like properties of electrons. The position and momentum of an electron cannot both be determined simultaneously. The region in space around the nucleus in which an electron is most probably located is what can be predicted for each electron in an at ...
... different elements is a direct consequence of wave-like properties of electrons. The position and momentum of an electron cannot both be determined simultaneously. The region in space around the nucleus in which an electron is most probably located is what can be predicted for each electron in an at ...
down
... 2.1 What determines if a system needs to be described using Q.M? When do we use a particle description(classical) of an atomic or molecular system and when do we use a wave (quantum mechanical) description? two criteria are used! 1) The magnitude of the wavelength of the particle relative to the ...
... 2.1 What determines if a system needs to be described using Q.M? When do we use a particle description(classical) of an atomic or molecular system and when do we use a wave (quantum mechanical) description? two criteria are used! 1) The magnitude of the wavelength of the particle relative to the ...
The Future of Computer Science
... Today, we don’t believe quantum computers can solve NP-complete problems in polynomial time in general (though not surprisingly, we can’t prove it) Bennett et al. 1997: “Quantum magic” won’t be enough If you throw away the problem structure, and just consider an abstract “landscape” of 2n possible s ...
... Today, we don’t believe quantum computers can solve NP-complete problems in polynomial time in general (though not surprisingly, we can’t prove it) Bennett et al. 1997: “Quantum magic” won’t be enough If you throw away the problem structure, and just consider an abstract “landscape” of 2n possible s ...
2. Essential Chemistry
... o Cells constantly rearrange molecules by breaking existing chemical bonds and forming new ones o Such changes in the chemical composition of matter are called chemical reactions o Chemical reactions enable atoms to give up or acquire electrons in order to complete their outer shells These interac ...
... o Cells constantly rearrange molecules by breaking existing chemical bonds and forming new ones o Such changes in the chemical composition of matter are called chemical reactions o Chemical reactions enable atoms to give up or acquire electrons in order to complete their outer shells These interac ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).