Electron physics
... Electron charge (e) = 1.6x10 C Speed of light (c) = 3x108 ms-1 1. In an electron gun in which direction do the electrons travel – cathode to anode or anode to cathode? 2. If the field is uniform for the majority of the distance between the cathode and anode what can you say about the velocity of the ...
... Electron charge (e) = 1.6x10 C Speed of light (c) = 3x108 ms-1 1. In an electron gun in which direction do the electrons travel – cathode to anode or anode to cathode? 2. If the field is uniform for the majority of the distance between the cathode and anode what can you say about the velocity of the ...
Linear Transformations and Matrix Algebra
... Rn , n ≥ 4 these tools are no longer available. However, we would still like to have similar results to those of Rn , n = 2, 3. To make a long story short, we will have these results for arbitrary vectors in Rn but not immediately. The first thing we must do is show that |x · y| ≤ |x||y|, which is k ...
... Rn , n ≥ 4 these tools are no longer available. However, we would still like to have similar results to those of Rn , n = 2, 3. To make a long story short, we will have these results for arbitrary vectors in Rn but not immediately. The first thing we must do is show that |x · y| ≤ |x||y|, which is k ...
... III. Credit Hours; Three IV. Objectives of the Course; This course is intended to give the student an understanding of the historical development of modern physics and the Schroedinger formulation of quantum mechanics. The student will learn to apply this methodology to simple physical systems such ...
Abstract Submitted for the MAR12 Meeting of The
... Coupling quantum microwave circuits to quantum optics via cavity electro-optic modulators1 MANKEI TSANG, National University of Singapore — Experimental circuit quantum electrodynamics has made great strides in recent years, but it remains an open question how the quantum information stored in the m ...
... Coupling quantum microwave circuits to quantum optics via cavity electro-optic modulators1 MANKEI TSANG, National University of Singapore — Experimental circuit quantum electrodynamics has made great strides in recent years, but it remains an open question how the quantum information stored in the m ...
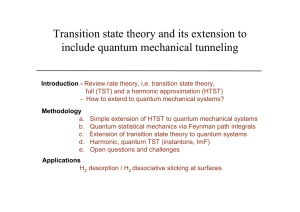
Transition state theory and its extension to include quantum
... “In view of [its] success, it is unfortunate that the theory [TST] does not enjoy a better understanding and confidence among non-specialists. Some of this difficulty can be traced to the rather unconvincing derivations of the [TST] expression for the rate constant which are found in many physical c ...
... “In view of [its] success, it is unfortunate that the theory [TST] does not enjoy a better understanding and confidence among non-specialists. Some of this difficulty can be traced to the rather unconvincing derivations of the [TST] expression for the rate constant which are found in many physical c ...
1.1 Construction of two band models
... For this Hamiltonian, we can see that linear coupling appears naturally between and orbitals. However, and orbitals are degenerate with each other at and there are three bands appearing in low energy physics for this system. To remove this degeneracy, we consider a coupling to the angular momentum o ...
... For this Hamiltonian, we can see that linear coupling appears naturally between and orbitals. However, and orbitals are degenerate with each other at and there are three bands appearing in low energy physics for this system. To remove this degeneracy, we consider a coupling to the angular momentum o ...
review outline - Michigan State University
... Ionic compounds are overall electrically neutral compounds formed by the combination of anion and cation o Li+ N3- Li3N o Co2+ Cl- CoCl2 o Cr4+ O2- CrO2 ...
... Ionic compounds are overall electrically neutral compounds formed by the combination of anion and cation o Li+ N3- Li3N o Co2+ Cl- CoCl2 o Cr4+ O2- CrO2 ...
BLP presentation
... • Electron is a shell of electric charge surrounding the proton nucleus (or a positron). • Can be modeled as an infinite number of infinitesimal sized charge currents that orbit on circular paths (“great circles”) around the proton (or around the positron). • The transition state orbitsphere (TSO) i ...
... • Electron is a shell of electric charge surrounding the proton nucleus (or a positron). • Can be modeled as an infinite number of infinitesimal sized charge currents that orbit on circular paths (“great circles”) around the proton (or around the positron). • The transition state orbitsphere (TSO) i ...
PPT - Henry Haselgrove`s Homepage
... At the moment, our bound on the energy gap becomes very weak when you make the system very large. Can we improve this? ...
... At the moment, our bound on the energy gap becomes very weak when you make the system very large. Can we improve this? ...
arXiv:0911.1876 - Harvard University
... quantized conductance (Quantum Hall systems, Quantum Spin Hall Sysytems) fractional charges (Fractional Quantum Hall systems, Polyethethylene) ...
... quantized conductance (Quantum Hall systems, Quantum Spin Hall Sysytems) fractional charges (Fractional Quantum Hall systems, Polyethethylene) ...
Atom-notes-Powerpoint-upload
... in the nucleus of an Atom. Nucleus: It’s where the Protons and Neutrons are located in an Atom. Protons: Positively Charged Particles in the Nucleus of the atom. Mass = (approx) 1 AMU Neutrons: Neutrally charged particles in the nucleus of an atom Mass = (approx) 1 AMU Mass Number of an atom: Number ...
... in the nucleus of an Atom. Nucleus: It’s where the Protons and Neutrons are located in an Atom. Protons: Positively Charged Particles in the Nucleus of the atom. Mass = (approx) 1 AMU Neutrons: Neutrally charged particles in the nucleus of an atom Mass = (approx) 1 AMU Mass Number of an atom: Number ...
Proton Kinetic Energy Maxima in Stable and Supercooled Liquid
... of this system in biological and physical sciences [1]. This simple liquid still represents a challenging puzzle [2–4]. A crucial piece of information is the understanding of the local environment of the protons which plays a fundamental role in hydrogen bonding. Recent studies on the microscopic st ...
... of this system in biological and physical sciences [1]. This simple liquid still represents a challenging puzzle [2–4]. A crucial piece of information is the understanding of the local environment of the protons which plays a fundamental role in hydrogen bonding. Recent studies on the microscopic st ...
syllabus.pdf
... (a) Eigenstate-Eigenvalue Link (This is what Fine [Fin87] calls the “rule of silence” and “rule of law.”); Collapse of the Wavefunction (b) Booleanism (c) The problem of the non-maximal observable (d) Definability and the Bub-Clifton theorem [BC96] 8. What is the status of the other quantities? (a) ...
... (a) Eigenstate-Eigenvalue Link (This is what Fine [Fin87] calls the “rule of silence” and “rule of law.”); Collapse of the Wavefunction (b) Booleanism (c) The problem of the non-maximal observable (d) Definability and the Bub-Clifton theorem [BC96] 8. What is the status of the other quantities? (a) ...
Implementations of Quantum Information
... Trapped Ions The trapped ion system is an early and promising medium for realizing quantum information processing. Ions are charged atoms, and electric fields are used to confine or move these ions in a lattice. Quantum information is encoded in the electron energy level. Coupling is obtain ...
... Trapped Ions The trapped ion system is an early and promising medium for realizing quantum information processing. Ions are charged atoms, and electric fields are used to confine or move these ions in a lattice. Quantum information is encoded in the electron energy level. Coupling is obtain ...
2012 - University of Utah Physics
... chain), which converts hydrogen nuclei (protons p) into helium nuclei (4 He). The overall reaction can be thought as that every four protons are converted into one 4 He nucleus, with an energy release Q. The masses of proton and 4 He nucleus are mp = 1.0076mu and m4 He = 4.0026mu , respectively, whe ...
... chain), which converts hydrogen nuclei (protons p) into helium nuclei (4 He). The overall reaction can be thought as that every four protons are converted into one 4 He nucleus, with an energy release Q. The masses of proton and 4 He nucleus are mp = 1.0076mu and m4 He = 4.0026mu , respectively, whe ...
EP-307 Introduction to Quantum Mechanics
... We Observe that from the final SG Z there are two beams Emerging No way to explain as Sz- was blocked Only conclusion we can draw is that the second Measurement disturbed the first measurement ...
... We Observe that from the final SG Z there are two beams Emerging No way to explain as Sz- was blocked Only conclusion we can draw is that the second Measurement disturbed the first measurement ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).