* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Exam 3 Stats
Evolution of metal ions in biological systems wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Signal transduction wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Peptide synthesis wikipedia , lookup
Interactome wikipedia , lookup
Genetic code wikipedia , lookup
Western blot wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Biosynthesis wikipedia , lookup
Point mutation wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Homology modeling wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Anthrax toxin wikipedia , lookup
Metalloprotein wikipedia , lookup
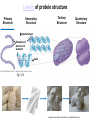
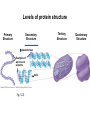
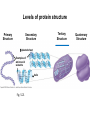
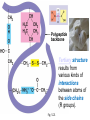
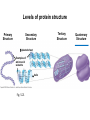
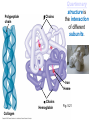
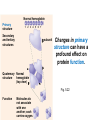
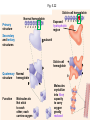
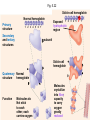
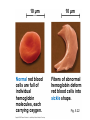
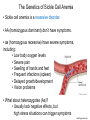
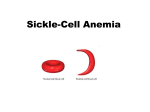
Proteins – Monomer: Amino acids – Polymer: Polypeptide (aka protein) – Key Elements: C, H, N, “R” (R varies) Proteins are: a) Hydrophobic b) Hydrophilic c) Could be either d) Not sure Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Protein Functions • Proteins are >50% of the dry mass of most cells – Structural support – Storage – Transport – Cellular communications – Movement – Defense against foreign substances – ALL enzymes are proteins – chemical reactions wouldn’t occur in our cells without proteins! Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Functions of Proteins: Examples Proteins have many diverse functions; they are the most functionally diverse type of macromolecules 1. Structural support (e.g. silk of spider webs) 2. Storage of energy & nitrogen (e.g. egg albumin) 3. Transport of substances within organisms (e.g. hemoglobin) or across membranes (e.g. aquaporins) 4. Signaling: long-distance (e.g. insulin) or short-distance; gene-regulatory proteins; receptor proteins Functions of Proteins: Examples Proteins have many diverse functions; they are the most functionally diverse type of macromolecules 5. Defense against invading pathogens (e.g. antibodies in immune system) Antibody protein Protein from flu virus 6. Movement (e.g. muscle proteins) ATP Actin and Myosin 7. Metabolic catalysts (enzymes) Enzymes speed up chemical reactions Fig. 8.16 Essential amino acids Fig. 41.2 Methionine Valine Beans and other legumes Threonine Phenylalanine Leucine Corn (maize) and other grains Isoleucine Tryptophan Lysine So, a diet of only wheat bread and corn would not be sufficient. Peptide bond is C-N bond between neighboring amino acids formed by removal of H2O (dehydration synthesis). Peptide bond Side chains Peptide bond Since amino acids in proteins are bonded together by peptide bonds, proteins are also called polypeptides. Backbone Fig. 5.18 Levels of protein structure Primary Structure Secondary Structure Tertiary Structure Quaternary Structure pleated sheet Examples of amino acid subunits helix Fig. 5.21 imagecent.com; alison.knitsmiths.us; bigtopshirtshop.com Primary Structure 1 Exact sequence of amino acids is called the primary structure of a protein. 5 10 Amino acid subunits 15 20 25 Fig. 5.21 Levels of protein structure Primary Structure Secondary Structure pleated sheet Examples of amino acid subunits helix Fig. 5.21 Tertiary Structure Quaternary Structure Secondary Structure pleated sheet helix Fig. 5.21 Secondary structure: Helix or pleated sheet. Pleated sheet protein often used for structural purposes Spider’s abdominal glands secrete silk fibers made of structural protein with pleated sheets. Fig. 5.21 Levels of protein structure Primary Structure Secondary Structure pleated sheet Examples of amino acid subunits helix Fig. 5.21 Tertiary Structure Quaternary Structure Polypeptide backbone Tertiary structure results from various kinds of interactions between atoms of the side chains (R groups). Fig. 5.21 Levels of protein structure Primary Structure Secondary Structure pleated sheet Examples of amino acid subunits helix Fig. 5.21 Tertiary Structure Quaternary Structure Polypeptide chain Chains Quarternary structure is the interaction of different subunits. Iron Heme Chains Hemoglobin Collagen Fig. 5.21 Normal hemoglobin Primary structure Val His Leu Thr Pro Glu Glu 1 2 3 4 5 7 6 Secondary and tertiary structures subunit Quaternary Normal hemoglobin structure (top view) Changes in primary structure can have a profound effect on protein function. Fig. 5.22 Function Molecules do not associate with one another; each carries oxygen. Fig. 5.22 Sickle-cell hemoglobin Normal hemoglobin Primary structure Val His Leu Thr Pro Glu Glu 1 2 3 4 5 7 6 Secondary and tertiary structures Val His Leu Thr Pro Val Glu Exposed 1 hydrophobic region 2 Sickle-cell hemoglobin 4 5 6 7 subunit Quaternary Normal hemoglobin structure Function 3 Molecules do Not stick to each other; each carries oxygen Molecules crystallize into fiber; capacity to carry oxygen greatly reduced Fig. 5.17 Amino acid R (rest) groups Nonpolar R groups: hydrophobic Glycine Alanine Valine Electrically Charged R groups: hydrophilic Aspartic acid Glutamic acid Lysine Leucine Arginine Isoleucine Histidine Fig. 5.22 Sickle-cell hemoglobin Normal hemoglobin Primary structure Val His Leu Thr Pro Glu Glu 1 2 3 4 5 7 6 Secondary and tertiary structures Val His Leu Thr Pro Val Glu Exposed 1 hydrophobic region 2 Sickle-cell hemoglobin 4 5 6 7 subunit Quaternary Normal hemoglobin structure Function 3 Molecules do Not stick to each other; each carries oxygen Molecules crystallize into fiber; capacity to carry oxygen greatly reduced 10 µm Normal red blood cells are full of individual hemoglobin molecules, each carrying oxygen. 10 µm Fibers of abnormal hemoglobin deform red blood cells into sickle shape. Fig. 5.22 The Genetics of Sickle Cell Anemia • Sickle cell anemia is a recessive disorder. • AA (homozygous dominant) don’t have symptoms. • aa (homozygous recessive) have severe symptoms, including: • Low body oxygen levels • Severe pain • Swelling of hands and feet • Frequent infections (spleen) • Delayed growth/development • Vision problems • What about heterozygotes (Aa)? • Usually lack negative effects, but high stress situations can trigger symptoms Healthyyounow.com Why hasn’t natural selection eliminated sickle cell anemia? Some clues: • 1 in 10 African Americans is a carrier for sickle cell anemia • Rates of sickle cell anemia are also higher in people with Mediterranean, Middle Eastern, and Indian ancestry “Heterozygote Advantage” Fig. 23.17 Frequencies of the sickle-cell allele Distribution of malaria caused by Plasmodium falciparum (a parasitic unicellular eukaryote) 0–2.5% 2.5–5.0% 5.0–7.5% 7.5–10.0% 10.0–12.5% >12.5% Sickle-cell disease inheritance. http://en.wikipedia.org/wiki/Sickle-cell_disease • Individuals with two copies of the altered gene suffer many complications (pain, infections, stroke, etc.), but are resistant to malaria. •Individuals with one copy suffer few complications and are also resistant. Fig. 5.17 Amino acid R (rest) groups Nonpolar R groups: hydrophobic Glycine Alanine Valine Electrically Charged R groups: hydrophilic Aspartic acid Glutamic acid Lysine Leucine Arginine Isoleucine Histidine Today’s Exit Ticket The bonds creating the primary structure of a protein are called 1)___________ and form between a 2)___ atom in one amino acid and a 3)____ atom in another amino acid. The bonds creating the secondary structure of a protein are called 4)__________ and form between 5)___________. The bonds creating the tertiary structure of a protein can be covalent, ionic, or hydrogen bonds, and form between 6)_______________. 7) Describe the quaternary structure of a protein.