* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Types of Weathering
Survey
Document related concepts
Water splitting wikipedia , lookup
Electrolysis of water wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Water pollution wikipedia , lookup
Metalloprotein wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Biochemistry wikipedia , lookup
Acid strength wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Acid–base reaction wikipedia , lookup
Transcript
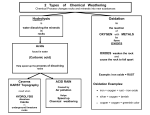
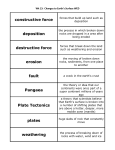
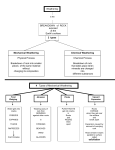
Types of Weathering Kim Bennison Katy Hogan Alex Gage Kelly Fischer Mechanical/Physical Weathering • • • • Exfoliation Ice Wedging Organic Wedging Abrasion Exfoliation When a rock has heat exerted upon it, along with pressure, it separates into layers. Ice Wedging When water leaks into the cracks of rocks, it freezes and causes the rock to crack. Organic Activity This occurs when burrowing animals, humans, or plants interfere and work their way into rocks. Abrasion Abrasion occurs when rocks rub against eachother and become rounded. Chemical Weathering • • • • Hydrolysis Oxidation Dissolution Acid – Carbonation – Precipitation – Plant Acid Hydrolysis When rocks sit in water for extended periods of time they begin to break down and have a clay-like texture. Oxidation When oxygen reacts with iron in rocks, they rust, taking on a red-orange color. Dissolution Rocks, when in water, react with acids in the water and dissolve. A clue that this has happened to a rock is the presence of small holes. Acid 1 • Carbonation- water absorbs carbon dioxide when rain falls or from decaying organic material. The carbon dioxide dissolved in water forms carbonic acid that reacts with many common minerals. Acid 2 • Precipitation- Water in the atmosphere absorbs sulfur oxides and nitrogen oxides. Through a series of chemical reactions these pollutants are converted into acids that are a cause of acid precipitation. Acid 3 • Plant Acid- When plants decay they release acids that react with the minerals in rocks.