* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Soil Biology and Biochemistry
Survey
Document related concepts
Fatty acid synthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Proteolysis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Metalloprotein wikipedia , lookup
Butyric acid wikipedia , lookup
Point mutation wikipedia , lookup
Protein structure prediction wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Peptide synthesis wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Genetic code wikipedia , lookup
Biochemistry wikipedia , lookup
Transcript
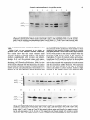
Soil Biol.Biochem.Vol. 26, No. 1, pp. 51-63, 1994 Copyright 0 1994 ElsevierScienceLtd Printed in Great Britain. All rights reserved 0038-0717/94$6.00+ 0.00 Pergamon CHARACTERIZATION OF TWO AROMATIC AMINO ACID AMINOTRANSFERASES AND PRODUCTION OF INDOLEACETIC ACID IN AZOSPIRILLUM STRAINS B. E. BACA, L. SOTO-URZUA, Y. G. XOCHIHUA-CORONAand A. CUERVO-GARCIA Department0 de Investigaciones Microbiologicas, Instituto de Ciencias, Universidad Puebla, Apartado Postal 1622, 72000 Puebla Pue, Mexico Autonoma de (Accepted 30 May 1993) Sunnnary-Experiments were made to quantify indole-3-acetic acid (IAA) excreted by different wild type strains of Azospirilhun spp. The microorganisms produce IAA, during the late stationary growth phase and show a significant increase in IAA production when tryptophan is present in the medium. Under these conditions with the A. brusilense strain UAP 14, we observed two aromatic amino acid aminotransferases. Their enzymatic activity and electrophoretic mobility under non-denaturating conditions were characterized using cell-free extracts. Biochemical studies showed that these two enzymes can be distinguished by their electrophoretic mobilities. These enzymes are constitutively produced and they are not repressed by tyrosine or by tyrosine plus phenlylalanine. described in Pseudomonas savastanoi and Agrobacterium tumefaciens. In Azospirillum this process is poorly understood. Hartman et al. (1983) and Zimmer et al. (1988) identified indole-3-pyruvic acid (IPyA), indole-3-acetaldehyde, and IAA as the substances that are excreted by A. brasilense Sp7 strain, suggesting that the transamination pathway is present in A. brusilense. Ruckdaschell et al. (1988) described four amino acid aminotransferases from A. lipoferum. We have found that Azospirillum spp wild type strains produced IAA and we demonstrated the presence of two aromatic amino acid aminotransferases associated with the production of IAA in A. brasilense strain UAP 14. INTRODUCTION Soil bacteria of the genus Azospirillum live in association with the root zones of grasses and other plants, among which are the economically-important cultivated plants maize, wheat, barley, rye, oat and rice. Reports indicate that Azospirillum can stimulate the growth of the host plants (Elmerich, 1984; Okon, 1985). The bacterium may affect plant growth by nitrogen fixation (Dobereiner and Pedrosa, 1987). Inoculation with Azospirillum may also improve the efficiency of applied mineral nutrients by helping the plant scavenge limiting nutrients (Lin et al., 1983). These effects have been proposed to be related to the production of plant growth regulators by Azospirillwn spp (Morgenstern and Okon, 1987). The production of phytohormones by the microorganism seems to play an essential role in the Azospirillum-plant interaction. It has been shown that bacterial production of the phytohormone IAA is involved in interactions between microorganisms and plants (Morris, 1986). In grasses, legumes and tomato plants, as tested in Petri dishes or pouch systems, inoculation with Azospirillum increased root diameter, density, and length of root hairs (Jain and Patriquin, 1984; Okon et al., 1988). Experiments with suitable concentrations of IAA produced, changes in root tip morphology comparable to those produced by Azospirillum, suggesting the involvement of auxin produced by Azospirillum on root morphology (Jain and Patriquin, 1985). Several pathways have been reported for the conversion of tryptophan to IAA by bacteria (Van Onckelen ef al., 1986). The most studied pathway involved conversion via indole-3-acetamide (IAM) MATERIALSAND METHODS Bacterial strains and growth conditions The A. brasilense H18, UAP 14, and A. lipoferum UAP 06 wild type strains used were obtained from Professor J. Caballero-Mellado (Universidad Autonoma de Puebla, Mexico) and A. brasilense AZ 30 INTA was supplied by Professor E. A. RodriguezCaceres. To estimate IAA production Azospirillum strains were grown in a minimal medium (Jain and Patriquin, 1985), amended with 0.1% of NH,Cl, or 0.1% of KNO, as a nitrogen source. The medium was supplemented with 100 pg ml-’ sterilized tryptophan, tyrosine or tyrosine plus phenylalanine as indicated; 50 ml of medium was inoculated with 100 ~1 ml-’ of 109cfu ml-’ of different Azospirillum strains and grown in a 250 ml flask, for 48, 72 or 96 h at 32”C, with shaking at 160rev min-r. To determine the aromatic amino acid aminotransferase activity, A. brasilense strain UAP 14 was grown in a 500 ml flask 57 58 B. E. BACA ~n~ining 250 ml of medium, inoculated with 2 ml of 1.6 x 109cfuml-‘, then samples were collected at different times as indicated, Bacterial growth was measured photometrically with a Klett Summerson (green filter) or by plating and viable cell counting. IAA determination Bacterial cultures were harvested by centrifugation at 10,OOOgfor 10 min at 4°C. The supernatants were extracted with ethyl-acetate (Tien et al., 1979). The ethyl-acetate extracts were evaporated to dryness at 39°C under vacuum and residue redissolved in 2 ml of methanol and the IAA ~n~ntration was determined by the Salkowski reaction (Stahl, 1969). Cell-fee extracts preparation Culture of A. brasilense strain UAP 14 were harvested at 48, 72 or 96 h by centrifugation at 10,OOg for 10min at 4°C. Subsequent procedures were also made at 4°C. The cells (2 g wet wt) were washed in 10 ml (3 M) NaCl. After being stirred for 1 h the cells were centrifuged at 10,000 g for 10 min; the supernatant was discarded and the cells were resuspended in 20 ml of TES buffer pris-NC1 (SOmM, pH 8.5), EDTA, 50mna; NaCl, 50m~] and lysed with lysozyme (5~~grnl-‘) the mixture was stirred for 18 h, then centrifuged at 10,000g for 20 min. The supematant which is referred to as crude extract, was brought to 30% saturation with solid ammonium sulfate. After stirring for JOmin, the sample was centrifuged at 10,OOOgfor 10min. The supernatant was brought to 70% saturation with solid ammonium sulfate. After stirring and centrifugation as before the pellet was resuspended in 2 ml of a buffer [Tris-HCI (50 mhr, pH 8.5); dl-dithiothreitol (DTT), 1 mM, MgCl,. 7H,O, IOmM, pyridoxal phosphate, lOmhr, sodium azide, 0.02% and glycerol, lo%]. The enzyme solution was stored at 4°C. Enzyme activities assays Tyrosine aminotransferase and phenylalanine aminotransferase activities were determined by measuring the p-hydroxyphenyl pyruvate @HPP) and phenyl pyruvate (PP), produced using the procedure of Diamondstone (1966). The reaction temTable 1. IAA production et al. perature was 45°C. The reaction was stopped with (5 M) KOH and the amount of pHPP produced from tyrosine was estimated by its absorbance at 331 nm. Production of IPyA was measured following the method of Truelsen (1972). The reaction temperature was 45°C. The formation of IPyA was measured at 328 nm. Polyacrylamide gel electrophoresis and aromatic amino acid aminotransferase activity staining Electrophoretic analyses under non-denaturing conditions was done in a discontinuous system, the running buffer being T~sglycine (25 rn&&and 192 M, pH 8.3). The running gel consisted of 10% acrylamide and the stacking gel was made from 4% acrylamide. All procedures were done at 4°C. Protein (100 pg) extract was loaded in each well and tetrazolium dye reduction was used to stain aromatic amino acid aminotransferase activity. Gels were stained at room temperature in the dark. The staining solution contained: Tris-HCl buffer (0.1 M, pH 8.6); tryptophan or another L-amino acid, 5.5 mM; pyridoxal phosphate, 0.2 mM; phenazine methosulfate, 98 F”M; 2-oxoglutaric acid, 12.5 mM; and nitroblue tetrazolium, 0.6 mh9. Protein ~terminations Protein was determined by the method of Lowry et al. (1951) using bovine serum albumin as a standard. RESULTS Determination of IAA in the culture medium of Azospirillum We studied IAA accumulation in the culture medium produced by Azospiri~lum wild type strains, to understand the influence of nutrient conditions on the synthesis of ph~oho~one by these microorganisms. IAA production was influenced by the nutrient content of the medium and by the incubation time as shown in Table 1. IAA produced by Azospirillum was released continuously, especially when the bacteria were grown in medium supplemented by the amino by AzospiriNum spp wild type strains pg MA ml-’ of the culture medium* KNO,tb NH&it’ KNO,-Trpd NH&l-Trp’ 48 72 96 48 72 96 48 72 96 48 72 9s 38 54 39 25.5 25 45 30 64 strains A. iipofenun UAP 06 A. bradense AZ 30 INTA 0.02 0.07 0.15 ND$ 0.15 1.1 0.27 313 33.1 7 0.04 0.02 0.04 ND 0.2 0.88 0.04 5.3 H18 UAP 14 0.03 0.16 0.05 0.4 0.27 0.8 ND ND 0.15 0.1 0.61 0.13 4.3 15 27 48 29.7 33.5 63.5 34 23.5 5.5 lIAA concentration as measured with the Salkowski reaction. (Data represent the means of three experimental determinations+ standard errors: ‘It5 x IO-’ to +0.015; b+0.04S to J20.2; ‘f0.56 to +4.3; df 1.2 to 6.7.) t’fhe medium contained either 260 mg N I-’ as NH,CI or 140 mg N I-’ as KNO,. fND is not determined. $Hours of incubation. Aromatic aminotransferase in Azospirillum strains 59 (l.OOOE+ 08) 250 225 (LOOOE + 07) (l.OOOE+ 06) (l.OOOE+ 05) 15 (l.OOOE+ 04) 50 25 (l.OOOE+ 03) 0 5 10 15 20 25 30 35 40 0 50 45 Hours Fig. 1. Growth curves of Azospiriffum spp strains were grown on Jain and Patriquin medium supplemented with 100pg ml-’ L-tryptophan. Log number of viable cells ml-‘: HI8 (*-*); AZ 30 INTA (O-0); 14 (O-0) A. lipoferum UAP 06 ( + - + ). Dotted lines absorbance as Klett units. IAA excreted in medium containing KNO, was about 2-10 fold (0.139-1.15 pgml-‘) as compared to IAA produced in medium supplemented with NH,Cl, in A. lipoferum UAP 06, A. brasilense AZ 30 INTA, and in A. brasilense H 18 strains, but this effect was not observed in A. brasilense strain UAP 14. A significant increase in IAA production was observed when the medium was supplemented with tryptophan, 5&100 fold higher (30-64 pg ml-‘) as compared to IAA produced in KNOj or NH,Cl supplemented medium. With the amino acid precursor added at the beginning, it was also observed that IAA liberation was stimulated within 48 h. Azospirillum wild-type strains grow at similar rates (Fig.1). Therefore, differences in IAA production are probably not due to non-normal growth (Table 1; Fig. 1). acid precursor. Kinetics of ZAA production and spectfk activity of aromatic amino acid aminotransferases (AAT)from A. brasilense strain UAP 14 Previous data have suggested that IAA biosynthesis in Azospirillum could occur through an IPyA intermediate derived from tryptophan by an aminotransferase activity (Hartmann et al., 1983; Zimmer, et al., 1988). To examine this biosynthetic pathway we investigated IAA production from A. brasilense strain UAP 14 grown at four different growth conditions for 72 h [Fig. 2 (A)]. The IAA excreted by A. brasilense UAP 14 varied from 0.05 to 2.6 pg ml-‘, when cultured in a medium supplemented with tyrosine, phenylalanine plus tyrosine or without amino acid. Under such growth conditions IAA production was almost the same, when compared on the basis of biomass as determined as wet weight [Fig. 2 (A) and (C)l. However, IAA production increased from 1.6 to UAP 50pgml-’ with the addition of tryptophan to the medium. Aminotransferase activities were determined in crude extracts and in precipitated extracts (30-70% of ammonium sulfate saturation) [Fig. 2 (C)l. Tryptophan was used as an aromatic amino acid donor, and 2-oxoglutaric acid as the amino group acceptor in the reaction mixture. Specific activities in extracts from cultures without amino acids ranged from 16 to 22 nmol of IPyA mgg’ protein ml-‘. However, in the presence of amino acids added to the culture medium, the specific activities ranged from 27 to 38 nmoles of IPyA mg-’ protein ml-‘. Polyacrylamide gel electrophoresis matic amino acid aminotransferase (PAGE) of aro- When protein extracts from cultures grown under four different nutrient conditions were subjected to electrophoresis on non-denaturing gels and were stained for AAT activity two bands were detected (Fig. 3) both convert tryptophan to IPyA in the presence of 2-oxoglutaric acid. Omitting either tryptophan or 2-oxoglutaric acid from the mixture reaction completely eliminated the two bands. Substitution for tryptophan by either tyrosine or phenylalanine [Fig. 4 (B)] revealed the same two activity bands. Only the large band showed aminotransferase activity when histidine was used as amino donor [Fig. 4 (C)l. however, the two AAT isoenzymes did not show aminotransferase activity when valine, isoleucine or aspartate (Fig. 5) were used as donor substrates. Protein extracts derived from cultures grown with tryptophan, tyrosine or tyrosine plus phenylalanine tested for AAT activity showed two isoenzymes pattern (Figs 3 and 4). This result indi- B. 60 E. BACAef a/. cates that both of these two AATs are not regulated by the aromatic amino acid added to the medium. DISCUSSION We have studied the excretion into culture medium of IAA by Azospirilium spp, in order to know the influence of nutrient conditions on the synthesis of phytohormones. The data obtained showed that the presence of KNO, has little effect on IAA production as compared with NH,CI. Tien et al. (1979), also reported that combined N has little effect on the production of IAA by A. bradense. Moreover wild-type strains excreted IAA in stationary phase culture. This concentration of IAA (20-88 ng ml-‘) is within a physiologicaIly active range that may affect plants roots (Plazinski and Roife, 985). Also this production is further stimulated by the addition of tryptophan to the medium. Both species produce large quantities of IAA in the presence of an amino acid precursor. These data agree well with those communicated by others for the A. brasilense Sp7 strain (Tien et al., 1979; Jain and Patriquin, 1985). In our tests A. brasilense strain IJAP 14 was the best IAA producer; it is interesting to note that this bacte~um was isolated from rhizosphere soil Azospiri~lum IAA production from A. brasilense UAP 14 strain 60 55 (A) F 0 12 24 36 48 60 72 Hours Biomass wet weight A. h&lease lor 0 UAP 14 strain Specific activity AATs A. brasiiense UAP 14 strain W 12 24 36 Hours 48 60 72 0 12 24 36 48 60 Hours Fig. 2. (A) IAA production of A. brdense strain.UAP 14grown in a medium supplemented with tyrosine (*--‘); tryptophan (O-0); tyrosine plus phenylalanine ( + - + ); or without amino acid O-J-_O). (B) Biomass as wet weight strain UAP 14 grown under same nutrient conditions. (C) Total specific activity of aromatic amino acid aminotransferase, one unit catalyzes the formation of I PMof IPyA mg-’ protein ml-‘, media conditions tyrosine (*-*); tryptophan (C-0); tyrosine plus phenylalanine ( + - + ); or without amino acid (O-c]). 72 Aromatic aminotransferase in Azospirillum strains 61 AATt AATZ Fig. 3. Non-denaturing PAGE of AATs from cell-free extracts of A. brusilense strain UAP 14. Nutrient conditions: cultures grown without amino acids (wells 1 and 2 ); with tryptophan (wells 3 and 4); tyrosine (wells 5 and 6); tyrosine plus phenylalanine (wells 7 and 8). Wells 1, 3, 5 and 7 were crude extracts; wells 2,4,6 and 8 were 30-70% ammonium sulfate fractions. Gel stained with tryptophan as amino acid donor. from a cactaceous plant (Mascarua-Esparza et al., 1988). Strain UAP 14 was compared in its ability to produce IAA in four different nutrient conditions. Our results show that this strain released small amounts of IAA in minimal medium, and minimal medium supplemented with tyrosine and phenylalanine. In E. coli, the aromatic amino acid aminotransferase is repressed by tyrosine (Gelfand and Steinberg, 1977; Powell and Morrison, 1978). In view of the results obtained with this bacterium, the amino acid tyrosine was chosen because it could presumably inhibit tyrosine and phenylalanine biosynthesis as is done by E. cd, and, therefore, diverts chorismic acid A AAT, -“, AAT, - m 1 2 3 4 to the endogenous synthesis of tryptophan. However the amounts of IAA production were similar to those obtained in the absence of either tyrosine or tyrosine plus phenylalanine. On the other hand, large amounts of IAA were present in the culture medium when the bacterium UAP 14 was grown in the presence of tryptophan. To know if aromatic amino acid aminotransferase (AAT) could be involved in biosynthesis of IAA we studied and identified aminotransferase activity that reacted with tryptophan in crude extracts and the ammonium sulfate fractions. The enzymatic activity was little affected by the type of aromatic amino acid added to culture medium. Indeed a slight increase in the specific activity was observed when 5 6 7 8 9 10 11 12 .’ ,. Fig. 4. Non-denaturing PAGE of AATs from cell-free extracts of A. brasdense strain UAP 14. Nutrient conditions: cultures grown with tyrosine. Gel A, wells 1 and 2; cells harvested at 18 h; wells 3 and 4; 24 h; wells 5 and 6; 36 h; wells 7 and 8; 48 h; wells 9 and 10; 72 h. Gel stained with tryptophan as amino acid donor; wells 11 and 12 same as 9 and 10 but stained without amino acid donor as a negative control. Gel B, cells harvested at 18, 24, 36, 48, or 72 h respectively and the gel stained with tyrosine as amino acid donor. Gel C, the cell-free extracts as same as gel B but stained with histidine as amino acid donor. 62 B. E. BACA etal. Fig. 5. Non-denaturing PAGE of AATs from extracts of Azospiriilum brasilense strain UAP 14. The nutrient conditions are the same as noted in Fig. 4. Cells harvested at 24,36,48 or 72 h respectively. Gel A, wells: 1, 24h; 2, 36 h; 3, 48 h, and 4, 72 h. Gel stained with valine as amino acid donor. Wells 5 and 6 the amino acid donor was omitted as a negative control. Wells 7 and 8 the amino acid donor was tryptophan. Gel B, stained with aspartic acid as amino acid donor; Gel C, stained with isoleucine as amino donor. tryptophan, tyrosine or tyrosine plus phenylalanine were added to the medium as compared with specific activity obtained in a minimal medium without amino acids. The addition of tryptophan to the medium (which promotes a dramatic increase in IAA synthesis of A. brasilense UAP 14 strain) did not show any significant effect on the aminotransferase activity. To identify the aminotransferase enzymes that react with tryptophan, crude extracts and ammonium sulfate fractions were run on PAGE under non-denatured conditions. Our data clearly demonstrated the presence of two aromatic amino acid aminotransferases (AATl, and AAT2). Only one of these two isoenzymes showed activity with histidine as substrate [Fig. 4 (C)J and might be involved in the catabolism of this amino acid. Both enzymes could be involved in IAA production. Neither of these enzymes show activity with branched chain amino acid or as an aspartate aminot~nsferase (Fig. 5). The A. brasilense A‘ATl enzyme unlike the E. coli enzyme (Powell and’Morrison, 1978), showed catalytic activity when histidine replaced tyrosine, phenylalanine or tryptophan as the amino acid donor. Similar activity has been detected for some AATs from R. leguminosarum (Perez-Galdona et al., 1989). We detected two other aminotransferase activities that used aspartate, valine and isoleucine as amino acid donors (Fig. 5, top arrows). Our work showed that unlike E. coli (Gelfand and Steinberg, 1977) and A. tumefaciens (Liu and Kado, 1979), AAT activity in A. brasilense strain UAP 14 is not affected by the presence of tyrosine in the growth medium. Indeed, data reported in Figs 3 and 4 clearly demonstrate that AATl and AAT activities are not repressed by tyrosine. These results suggested that both enzymes are constitutive. However, in cells grown with tryptophan, tyrosine, or tyrosine plus phenylalanine, total AAT activity had doubled. The inability of the aromatic amino acids (phenylalanine and tyrosine) to function as regulatory effecters for IAA biosynthesis suggests that IAA synthesis in A. brasilense may be not subjected to coordinated regulation of phenylalanine and tyrosine synthesis. These results suggest that IAA biosynthesis could be regulated by the tryptophan pool in cells. Acknowledgements-We thank Dr Miguel Lara F., Departamento de Biologia Molecular de Plantas, Institute de Biot~olo~a-UNAM, for helpful suggestions and critical reading of the manuscript. This work was partially supported by Subdireccion de Investigation Cientifica y Superacion Academica de la SEP. REFERENCES Diamondstone T. I. (1966) Assay of tyrosine transaminase activity by conversion of ~-hydroxyphenyIpyruvate to hydroxy~~aldehyde. A~aiyt~cal Eiochem~stry 16, 395-401. Dobereiner J. and Pedrosa F. 0. (1987) In Nitrogen FixingBacteria in Non-Leguminous Crop Piants. Brock Springer Series in Contemporary Bioscience. Science Tech., Madison. Elmerich C. (1984) Molecular biology and ecology of diazotrophs associated with non-leguminous plants. BiolTechnology 2, 961-978. Gelfand D. H. and Steinberg R. A. (1977) Escherichia cob’ mutants deiicient in the aspartate and aromatic amino Aromatic aminotransferase acid aminotransferases. Journal of Bacteriology 130, 429440. Hartmann A., Singb M. and Kligmuller W. (1983) Isolation and characterization of Azospirillum mutants excreting high amounts of indole acetic acid. Canadian Journal of ~icrobjolog~ 29, 916-923. Jain D. K. and Patriquin D. G. (1984) Root hair deformation, bacterial attachment, and plant growth in wheat Azospirillum associations. Avplied and Environmental bkobiology 48, 1208-1213. -. Jain D. K. and Patriauin D. G. (1985) Characterization of a substance produced by A~ospi~illum which causes branching of wheat root hairs. Canadian Journal of microbiology 31, 206-210. Lin W., Okon Y. and Hardy R. W. F. (1983) Enhanced mineral uptake by Zea mays and Sorghum bicolor roots inoculated with Azospirillum brasilense. Applied and En vironmenral Microbiology 45, 1775-1779. Liu S. T. and Kado C. I. (1979) Indoleacetic acid productioti a plasmid function of Agro~~~eri~ tu~fa&~ C58. Biochemi~aiand Bjophysical Research Co~~~cat~o~s 99, 171-178. Lowry 0. H., Rosebrough N. J., Farr A. L. and Randall R. J. (1951) Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry 193, 265-275. Mascarua-Esparza M. A. Villa-Gonzalez R. and CaballeroMellado J. (1988) Acetylene reduction and indoleacetic acid pr~uction by Azospiri~l~ isolates from eactaceous plants. Plant and Soil 106, 91-95. Morgenstern E. and Okon Y. (1987) The effect of Azospirillum brasilense and auxin on root morphology in Sorghum bicolor, Sorghum sudanense. Arid Soil Research and Rehabilitation 1, 115-127. Morris R. 0. (1986) Genes specifying auxin and cytokinin biosynthesis in p~yto~thogens. Annual Review of Plant Physiology 37, W-538. Okon Y. (1985) Azospirillum as a potential inoculant for agriculture. Trends in Biotechnology 3, 223-228. Okon Y.. Fallik E.. Sarie S.. Yahalom E. and Tal S. (1988) Plant growth prdmotiig effects of Azospirillum. In ktro: gen Fixafion: One Hundred Years After (H. Bothe, F. J. in A.zuspirillum strains 63 de Bruijn and W. E. Newton, Eds), pp. 741-745. Fisher, Stuttgart. Perez-Galdona R., Corzo J., Leon Barrios M. and GutierrezNavarro A. M. (1989) Aromatic amino acid aminotransferases in ~~~o~~urn’le~os~~ biovar t&&ii their role in indoleacetic acid synthesis. So17Biology & Biochemistry 21, 573-579. Planzinski J. and Rolfe B. G. (1985) Interaction of Arospirillum and Rhizobium strains leading to inhibition of nodulation. Applied and Environmental Microbiology 49, 990-993. Powell J. T. and Morrison J. F. (1978) The purification and properties of the aspartate aminotransfera~ and aromatic acid aminotransferase from Escherichia coli. European Journal of Biochemistry 87, 391-400. Ruckdaschel E., Lewis-Kittell B., Helinski D. R. and Klingmuller W. (1988) Aromatic amino acid aminotransferase of Arospirillum hpoferum and their possible involvement in IAA biosynthesis. In Azospirillum IV Genetics, Physiology, Ecology (W. Klingmuller Ed.), pp. 49-53 Springer, Berlin. Stahl E. (1969) Thin-Layer Chromatography. Springer, Berlin. Tien T. M., Gaskins M. H. and Hubbell D. H. (1979) Plant growth substances produced by Arospirillum brasilense and their effect on the growth of pearl-millet (Pennisetum americanurnL.). Applied and EnvironmenI~ Microbiology 37, 10161024. Truelsen T. A. (1972) Indole-3-p~u~c acid as an intermediate in the conversion of tryptopban to indole-3-acetic acid. I. Some characteristics of tryptophan transaminase from mung bean seedlings. Physiology Plantarum 26, 289-295. Van Onckelen H., Prinsen E., Inze D., Ruidelsheim P., Van Lijsebettens M., Follin A., Van Montagu M. and De Greef J. (1986) A~obacferjum T DNA gene/code for tryptophan Zmonooxygenase activity in tobacco crown gall celis. FEBS Letters 198, 357-360. Zimmer W., Roeben K. and Bothe H. (1988) An alternative explanation for plant growth promotion by bacteria of the genus Azospirillum. Planta 176, 333-342.