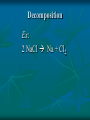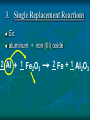* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Nuclear transmutation wikipedia , lookup
Cation–pi interaction wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Drug discovery wikipedia , lookup
Isotopic labeling wikipedia , lookup
Organic chemistry wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Chemical industry wikipedia , lookup
Acid–base reaction wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Atomic theory wikipedia , lookup
Asymmetric induction wikipedia , lookup
Marcus theory wikipedia , lookup
History of chemistry wikipedia , lookup
Water splitting wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Alkaline earth metal wikipedia , lookup
Process chemistry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Sodium hydroxide wikipedia , lookup
Homoaromaticity wikipedia , lookup
Metalloprotein wikipedia , lookup
George S. Hammond wikipedia , lookup
Rate equation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Electrolysis of water wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Sodium hypochlorite wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Electrochemistry wikipedia , lookup
Click chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Transition state theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
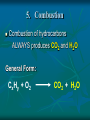
Chemical Reactions Chemical Reactions A. Definition Chemical reaction- process where one or more substances are rearranged to form new substances Precipitate Formation If you combine some substances, a reaction can occur forming a solid called a precipitate Gas formation Sometimes when two substances come in contact a reaction occurs producing a gas. Permanent color change Some substances, when combined, will turn another color Energy is produced in the form of light When sodium is placed in water, the reaction is so violent a fire results Temperature Change When some chemicals react they either: give off energy, exothermic reaction (feel warm to the touch) OR use energy, endothermic reaction, and get cooler C. Representing Chemical Reactions a) b) c) d) We use chemical equations as a way to represent reactions Substances that start the reaction are called reactants Substances that are formed are called the products Reactant 1 + Reactant 2 Product 1 + Product 2 (the number of reactants and products will vary) II. Describing Chemical Changes A. Writing equations Equations are more practical than words to express a chemical reaction It is very important that you note that some elements do not occur as single atoms when by themselves. (Ex. Cl2 & NaCl not NaCl2) A. Writing Equations If they are not combined with another element, they will bond with themselves, forming a diatomic molecule. In any chemical reaction, when you see these elements alone, they must be shown with a diatomic formula. Diatomic elements are always written as X2 when isolated in a reaction 2Mg + O2 2MgO Diatomic Elements Remember: 7 elements that NEVER occur as singular atoms (always paired with an the same or different element) H2 N2 O2 F2 Cl2 Br2 I2 -BrINClHOF- Ex: 2 HCl + K 2 KCl + H2 Br I N Cl HO F Bromine Iodine Nitrogen Chlorine Hydrogen Oxygen Fluorine Diatomics Br I N Cl HO F Anytime you are writing one of the Brinclhof elements in a reaction by itself, you must add the 2 subscript. Symbols used in reactions These symbols must be memorized! Symbol Meaning + combine with, reacts with yields, forms reversible reaction (s) solid (l) liquid (g) gas (aq) aqueous solution (dissolved in water) NOTE: All acids will be aqueous unless stated otherwise heat is supplied to the reaction precipitate is formed Precipitate = solid formed from two liquids gas is released pressure pressure applied Formula of the catalyst Catalyst Not in your notes! Word Equation equation expressed in words Iron (s) + chlorine (g) iron (III) chloride (s) Ex 1: Solid sodium hydrogen carbonate (bicarbonate) reacts with hydrochloric acid to produce an aqueous solution of sodium chloride, water and carbon dioxide gas. NaHCO3 (s) + HCl (aq) NaCl (aq) + H2O (l) + CO2 Equations that only show formulas of substances involved, but are not balanced are called skeletal equations Ex 2: Turn this into a sentence BaCl2 (aq) + Na2SO4 (aq) BaSO4 (s) + 2NaCl (aq) An aqueous solution containing 1 mole of Barium Chloride reacts with 1 mole of aqueous sodium sulfate to yield 1 mole of solid barium sulfate and 2 moles of aqueous sodium chloride. Practice Practice 1. Write equations for each chemical reaction. a. Sulfur burns in oxygen to form sulfur dioxide. S + O2 SO2 b. Heating potassium chlorate solid in the presence of the catalyst manganese (IV) oxide produces oxygen gas. Potassium chloride is left as a solid. KClO3(s) MnO2 O2 (g) + KCl(s) Practice Practice 2. Write a sentence that describes each chemical reaction. a. KOH(aq) + H2SO4(aq) H2O(l) + K2SO4(aq) An aqueous solution containing 1 mole of potassium hydroxide reacts with 1 mole of sulfuric acid to yield 1 mole of water and 1 mole of aqueous potassium sulfate. b. Na(s) + H2O(l) NaOH(aq) + H2(g) One mole of solid sodium reacts with 1 mole of water to yield 1 mole of aqueous sodium hydroxide and 1 mole of hydrogen gas. III. Balancing Chemical Equations A. Law of Conservation of Mass Mass can not be created or destroyed Since atoms have mass, the number of each type of atom must be equal on both sides of a reaction The sum of the mass of the reactant must equal the sum of the mass of products Rules for balancing equations Only coefficients are changed in balancing reactions Never change subscript during balancing The coefficient also tells you the number of atoms used in each compound When no coefficient is written it is assumed to be 1 Not in your notes! How many grams of solid silver are produced Cu(s) + AgNO3(aq) CuNO3(aq) + Ag(s) 88.4g 63.5g 148.3g 123.4g ? Ex 1: Write a balanced equation for the reaction of copper metal and an aqueous solution of silver nitrate to form aqueous copper (I) nitrate and silver. __ 1 Cu(s) + __ 1 AgNO3(aq) __ 1 CuNO3(aq) + __ 1 Ag Correct Formulas for: silver nitrate & copper (II) nitrate Ag+ NO3- Ag (NO3 ) Parentheses not needed Cu+2 NO3- Cu(NO3)2 Parentheses needed Cu(s) + AgNO3(aq) Cu(NO3)2 (aq) + Ag(s) Example 1 2 2 __ Cu(s)+ __ AgNO3(aq) __ Cu(NO3)2(aq)+ __ Ag 1 Cu 1 2 1 Ag 1 2 21 N 2 63 O 6 Balanced! Example 1 again 2 2 __ Cu(s)+ __ AgNO3(aq) __ Cu(NO3)2(aq)+ __ Ag 1 Cu 1 2 1 Ag 1 2 2 1 NO3 2 Balanced! Ex 2: Sodium and chlorine combine to form sodium chloride. 2 Na + 1 Cl2 2 NaCl Molar Ratio between sodium and chlorine is 2:1, it takes 2 moles of sodium to completely react with 1 mole of Chlorine (2 moles of sodium chloride are produced) Ex 2: Balance the following reaction: Na(s) + Cl2(g) 2 2 NaCl(s) 2 1 Na 1 2 2 Cl 1 2 Balanced! Molar Ratio between sodium and chlorine is 2:1, it takes 2 moles of sodium to completely react with 1 mole of Chlorine (2 moles of sodium chloride are produced) Ex 3: Sodium hydroxide and calcium bromide react to produce calcium hydroxide and sodium bromide. 2 NaOH + 1 CaBr2 1 Ca(OH)2 + 2 NaBr What is the molar ratio between sodium hydroxide and calcium bromide in this equation? 2:1 Tips for Balancing Chemical Reactions 1. Write a correct skeletal chemical equation (use oxidation number to make products) 2. Identify and Count the number of atoms of each element in the reactants and products. 3. Balance the elements one at a time by using coefficients. (NEVER CHANGE SUBSCRIPTS) it’s usually easier to begin with elements that appear only once save lone elements (i.e. O2) to balance last many times you can treat polyatomics as one unit Check each atom or polyatomic ion to be sure the equation is balanced 4. • • Sometimes it is helpful to think of water as HOH Finally, make sure all the coefficients are in the lowest possible whole number ratio that balances. Practice Practice 1. Write balanced chemical equations. a. Sodium and water form aqueous sodium hydroxide and hydrogen gas. Na + H2O(l) NaOH(aq) + H2(g) b. Aqueous calcium hydroxide and sulfuric acid react to form aqueous calcium sulfate and water. Ca(OH)2(aq) + H2SO4(aq) CaSO4(aq) + H2O(aq) Practice Practice 2. Balance the following reactions. a. 2SO2 + b. O2 2SO3 Fe2O3 + 3H2 2Fe + 3H2O c. 4P + 5O2 d. 2 1Al + P4O10 6HCl 2 AlCl3 + 3H2 3 e. 2C2H6 + 7O2 4CO2 + 6H20 IV. Types of Chemical Reactions A. Classifying Chemical Reactions There are 5 basic types: Synthesis Decomposition Single replacement Double Replacement Combustion 1. Synthesis Two or more substances combine to create a more complex substance (synthesis means “to make”) Two parts combine and make ONE product General Form: A + B AB Hey baby let’s get jiggy. Synthesis reaction of two elements Ex: 2 Al + ___ 3 Cl2 ___ 2 AlCl3 ___ Al 3+ 1Cl 2. Decomposition A complex substance is broken down into two ore more simpler substances (decompose means “to break down”) one substance breaks down into more than one simpler products General Form: AB A + B Break yoself fool! Decomposition Ex: 2 NaCl Na + Cl2 3. Single Replacement Reactions one element replaces another element in a compound to form new compound General Form: A + BX AX + B I’m gon’ dance with yo’ lady 3. Single Replacement Reactions Ex: aluminum + iron (III) oxide 2 Al + 1 Fe2O3 2 Fe + 1 Al2O3 4. Double Replacement Ions from two ionic compounds switch places Basically: an exchange of cations between two ionic compounds When a solution of sodium sulfate and barium chloride are mixed, the sulfate anion combines with the barium cation and this combination precipitates as barium sulfate. The newly formed sodium chloride remains soluble. Na2SO4(aq) + BaCl2(aq) BaSO4(s) + 2 NaCl(aq) 4. Double Replacement General Form: AB + CD AD + CB switch 4. Double Replacement 4. Double Replacement Ex: Li1+ I1- Ag1+ NO31- lithium iodide and aqueous silver nitrate react LiI + AgNO3 LiNO3 + AgI(s) 5. Combustion A substance combines with oxygen, releasing a large amount of energy in the form of light or heat On the reactant side, look for a hydrocarbon (a compound made of C and H) or an oxygenated hydrocarbon (a compound made of C, H and O), plus a diatomic oxygen. Basically, a compound reacts with O2 to produce energy, CO2 gas and a hydrocarbon 5. Combustion Combustion of hydrocarbons ALWAYS produces CO2 and H2O General Form: CxHy + O2 CO2 + H2O 5. Combustion Ex: Show combustion of propane (C3H8) gas 1 C3H8 + 5 O2 3 CO2 + 4 H2O I sell propane and propane accessories! V. Predicting Products from chemical reactions Synthesis: 1. Solid sodium reacts with chlorine gas. Diatomic Element Use oxidization #’s to make a formula unit ___ 2 Na(s) + ___ 1 Cl2(g) ___ 2 NaCl V. Predicting Products from chemical reactions Synthesis: 1. Solid zinc reacts with chlorine gas. Diatomic Element Use oxidization #’s to make a formula unit ___ 2 Zn(s) + ___ 1 Cl2(g) ___ 2 ZnCl V. Predicting Products from chemical reactions Decomposition: 1. Sodium Azide (NaN3) decomposes Separate Elements Diatomic atoms 2 NaN3(s) ___ 2 Na (s) + ___ 3 N2(g) ___ This is used in car air bags. V. Predicting Products from chemical reactions Decomposition: 2. Aluminum oxide decomposes. Al3+ Separate Elements O2Diatomic atoms 2 Al2O3(s) ___ 4 Al(s) + ___ 3 O2(g) ___ V. 1. Predicting Products from chemical reactions Single Replacement Reactions To predict products of SR reactions, create a new compound with the lone metal ion and the anion from the reactanct compound. The cation from the reacctant compound becomes a lone element on the product side. Identify the lone element as a cation or anion (by its oxidation number), and the anion and cation of the compound on the reactant side of the equation Ex 1: 2+ 1- Mg(s) + Zn(NO3)2(aq) Mg(NO3)2 + Zn Cation Anion V. 1. Predicting Products from chemical reactions Single Replacement Reactions The two cations will trade places. Use oxidation numbers to make the compound. Ex 1: 2+ 1- Mg(s) + Zn(NO3)2(aq) Mg(NO3)2 + Zn Cation Anion Not in notes! Problem Single Replacement Reactions Identify the lone element as a cation or anion (oxidation number), and the anion and cation of the compound on the reactant side of the equation F2 + NaBr Not in notes! Problem Single Replacement Reactions Identify the lone element as a cation or anion (oxidation number), and the anion and cation of the compound on the reactant side of the equation F2 + NaBr -1 +1 -1 Not in notes! Problem Single Replacement Reactions In this reaction the two anions switch place (remember + goes with a -) F2 + NaBr NaF + Br2 -1 +1 -1 Not in notes! Problem Single Replacement Reactions Balance F2 + 2NaBr 2 NaF + Br2 Ex 2: Mg(s) + Ag(NO3)(aq) +2 +1 -1 Switch: Mg(s) + Ag(NO3)(aq) Mg(NO3)2 + Ag Balance: Mg(s) + 2Ag(NO3)(aq) Mg(NO3)2 + 2Ag Practice 1. Write a balanced reaction for each SR reaction. a. Zn(s) + H2SO4(aq) ZnSO4 + H2 b. 2Na(s) + 2H2O(l) 2 NaOH + H2 HOH c. Sn(s) + 2NaNO3(aq) Sn(NO3)2 +2Na (use tin(II)) d. Cl2(g) + 2NaBr 2 NaCl + Br2 2. Double replacement reactions To predict products of a double replacement reaction, simply switch ions, being very careful to create new formulas from the new combinations (assume the constant oxidation #’s) Identify the anions and cations of both compounds (do not separate polyatomic ions!) Switch the cation of one compound with the cation of the other compounds. 2. Double replacement reactions Ex 1: Pb (NO3)2(aq) + KI(aq) +2 -1 +1 -1 USE OXIDATION NUMBERS TO FORM Switch: PRODUCTS Pb(NO3)2(aq) + KI(aq) PbI2 + K(NO3) Balance: 1Pb(NO3)2(aq) + 2KI(aq) 1PbI2 + 2K(NO3) 2. Double Replacement Reactions Remember, iron needs a roman numeral! Ex 2: FeS(s) + HCl (aq) +2 -2 +1 -1 Iron (II) Switch: FeS(s) + HCl (aq) FeCl2 + H2S Balance: FeS(s) + 2HCl (aq) FeCl2 + H2S 2. Double replacement reactions Ex 3: HCl(aq) + Na(OH) +1 -1 +1 -1 Switch: HCl(aq) + Na(OH) NaCl + HOH (or H2O) Balance: Practice 1. Write balanced equations for these DR reactions. a. NaOH + Fe(NO3)3 Na(NO3) + Fe(OH)3 b. 3KOH (aq) + H3(PO4) (aq) K3(PO4) + 3HOH c. NaCl + KBr NaBr + KCl 3. Combustion Reactions All combustion reactions will have products of CO2 & H2O. Ex 1: C2H4 + O2 ^All produce CO2 + H2O^ C2H4 + O2 CO2 + H2O Balance: C2H4 + 3O2 2CO2 + 2H2O