* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Opiate receptors, endogenous opioid systems in brain, Analgesia
Survey
Document related concepts
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Drug interaction wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
Drug design wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Theralizumab wikipedia , lookup
Dextropropoxyphene wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Psychopharmacology wikipedia , lookup
Transcript
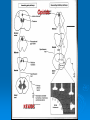
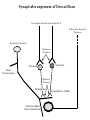
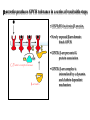
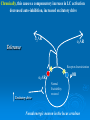
NeuroPharmacology (NB404): Dr. Charles Chavkin Professor of Pharmacology D425 HSB NeuroPharmacology (NB404): • How drugs interact with their targets. • How pharmacology can be used to discover new medicines. • How pharmacology can be used to increase our understanding of healthy and pathological brain functioning. Opiate receptors, endogenous opioid systems in brain, Analgesia, stress adaptation, drug addiction Natural opium alkaloids Morphine - gold standard Codeine Thebaine - (non-analgesic) Opiate chemical structures CH2=CH Naloxone CH3C=0 Heroin CH3C=0 Endogenous Opioid Agonists: enkephalin - 2 pentapeptides b-endorphin - POMC, ACTH dynorphin - endog kappa agonist NH2-Tyr-Gly-Gly-Phe-Leu-COOH Opioids NSAIDS SynapticArrangement of Dorsal Horn Descending Projections from R aphe' N. To Reticular Formation, Thalam us Dorsal Root Ganglion Excitatory Synapses Serotonin Serotonin From Nociceceptors Inhibitory Synapses Enkephalin Substance Pand other transmitters Dynorphin or GABA Types of Pain Nociceptive pain mechanical, thermal, chemical activation of nociceptors somatic pain: response to tissue injury inflammatory mediators: prostaglandins, substance P, bradykinin Neuropathic pain damage to nerves (trigeminal neuralgia, postherpetic pain, diabetic neuropathy) Actions of Opiates: analgesia anxiolytic sedation euphoria gut hypomotility (constipation) cough suppression respiratory depression pupillary constriction nausea and vomiting endocrine suppression itching (specifically morphine) Endogenous opioid peptides (enkephalins and b-endorphin) have Morphine-like effects Analgesia Euphoria Antidepressant Reduction in anxiety Endogenous opioids form important stress regulating systems in brain Endogenous dynorphin opioid peptides Analgesia Dysphoria Depressant ? Increase in anxiety ? Stress-induced analgesia Stress-induced dysphoria Stress-induced priming of relapse? QuickTime™ and a Motion JPEG OpenDML decompressor are needed to see this picture. Forced swim stress-induced analgesia is blocked by prodynorphin gene disruption Day 1 Day 2 Dyn KO mice generated by Hochgeschwender; see Sharifi et al., 1998 QuickTime™ and a YUV420 codec decompressor are needed to see this picture. Mechanisms of opiate actions: Activate mu (m) delta (d), or kappa (k) opioid receptors principal therapeutic opiates are selective for mu receptors Opioid receptors are members of the 7TM, G proteincoupled receptor superfamily (>1,000 members) Activation of opioid receptors inhibits neuronal activity increases potassium conductance decreases calcium conductance inhibits neurotransmitter release how do opiates act at a molecular level? GDP GTP PO4 GDP GDP GTP K+ Activated arrestin G-Receptor Kinase tolerance opioid dose Opiate Tolerance receptor desensitization compensatory adaptations in neuronal circuit learning mechanisms Physical Dependence compensatory adaptations in neuronal circuit Drug Withdrawal removal of opiate unmasks compensatory adaptations Drug Addiction (extremely rare during treatment of pain) b-arrestin produces GPCR tolerance in a series of resolvable steps • GPCR-PO4 activates b-arrestin • Newly exposed b-arr domain binds GPCR • GPCR- b-arr prevents Gprotein association G Protein receptor kinase b-arrestin • GPCR- b-arr complex is internalized by a dynamin and clathrin dependent mechanism Acutely, morphine inhibits LC firing - sedation Neuron hyperpolarized and NE release inhibited R K+ mOR Opiates inhibit Noradrenergic neuron in the locus ceruleus Chronically, this causes a compensatory increase in LC activation decreased auto-inhibition, increased excitatory drive R R Tolerance Receptor desensitization mOR R Excitatory drive Normal Excitability restored Noradrenergic neuron in the locus ceruleus Opioid withdrawal - abstinence syndrome Severity depends on dose used and rate of elimination. Rhinorrhea Lacrimation Chills Goose flesh - ‘cold turkey’ Muscle aches Diarrhea Yawning Anxiety Hostility Hyperalgesia Precipitated withdrawal by a partial agonist or antagonist administration Withdrawal R R Clonidine, an 2-adrenergic receptor agonist, is effective at reducing the sympathetic nervous system hyperactivity associated with acute opiate withdrawal. Opiate gone mOR R Hyper-Excitability state Excitatory drive Noradrenergic neuron in the locus ceruleus What is drug addiction? Correct use of prescribed medications for pain, anxiety and hypertension produce tolerance and physical dependence. Addiction is: compulsive drug use, obsessive thoughts about drug, use despite objective evidence of harm, loss of control of drug use, high risk of relapse once abstinent. Commonly Abused Prescription Opiates Buprenorphine (Buprenex, Subutex, Suboxone) Codeine Fentanyl (Actiq, Sublimaze, Duragesic) Hydrocodone (Vicodin, Vicoprofen) Hydromorphone (Dilaudid) Meperidine (Demerol) Methadone (Methadose, Dolophine) Morphine (MS Contin, Avinza, Oramorph SR) Oxycodone (OxyContin, Percocet, Percodan) Propoxyphene (Darvon) “Molecular Basis of Addiction” • Voluntary intake tolerance readily reversible physical dependence sensitization • Involuntary - compulsive intake cravings, obsession, self-destructive behavior Addiction - high relapse risk Progression to Addiction challenges at the molecular front: identify molecular and cellular changes in the addicted brain genes controlling risk of addiction molecular events controlling relapse risk Conditioned place preference measures the rewarding properties of drugs • assess drug craving Day: 1 2 3 4 5 6 Difference in time spent on drug-paired side (sec) Free Run, 30 min Forced swim stress exposure Cocaine, box 2, 30 min Vehicle, box 1, 30 min * 1200 Untreated mice, no stress Vehicle-treated FST mice nor-BNI treated FST mice 1000 800 600 * * 400 200 0 -200 Baseline Day 5 results Drug consumption Stress-induced priming of relapse? crash relapse time Heroin Cocaine Ethanol Nicotine stress Working Model - (wild speculation) Stress induces release of endogenous opioids in key brain regions (nAc and VTA). This results in ‘priming’ of the circuit - manifests as craving Activation of the endogenous kappa opioid system during the stress response elicits dysphoria, anxiety depression. Drug self-administration self medicates the depression. Kappa antagonists may be effective in treating this form of depression. SUMMARY: Opiates are important therapeutic tools Endogenous opioids have important role in mediating the adaptive response to stress Opiates can induce addiction - a compulsive use of drug despite adverse consequence