* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Thalamocortical neuron loss and localized astrocytosis in the Cln3
Cortical cooling wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Subventricular zone wikipedia , lookup
Neuroregeneration wikipedia , lookup
Neuroeconomics wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Multielectrode array wikipedia , lookup
Nervous system network models wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neurogenomics wikipedia , lookup
Neuroanatomy wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Haemodynamic response wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Aging brain wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neuroplasticity wikipedia , lookup
Synaptic gating wikipedia , lookup
Environmental enrichment wikipedia , lookup
Spike-and-wave wikipedia , lookup
Channelrhodopsin wikipedia , lookup
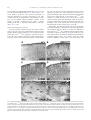
www.elsevier.com/locate/ynbdi Neurobiology of Disease 20 (2005) 823 – 836 Thalamocortical neuron loss and localized astrocytosis in the Cln3 Dex7/8 knock-in mouse model of Batten disease Charlie C. Pontikis,a,b Susan L. Cotman,c Marcy E. MacDonald,c and Jonathan D. Cooper a,b,* a Pediatric Storage Disorders Laboratory, Box P040, MRC Social Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, De Crespigny Park, King’s College London, London SE5 8AF, UK b Department of Neuroscience, Box P040, MRC Social Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, De Crespigny Park, King’s College London, London SE5 8AF, UK c Molecular Neurogenetics Unit, Center for Human Genetic Research, Massachusetts General Hospital, Charlestown, MA 02129, USA Received 3 April 2005; revised 13 May 2005; accepted 18 May 2005 Available online 11 July 2005 Juvenile neuronal ceroid lipofuscinosis (JNCL) is the result of mutations in the Cln3 gene. The Cln3 knock-in mouse (Cln3 Dex7/8 ) reproduces the most common Cln3 mutation and we have now characterized the CNS of these mice at 12 months of age. With the exception of the thalamus, Cln3 Dex7/8 homozygotes displayed no significant regional atrophy, but a range of changes in individual laminar thickness that resulted in variable cortical thinning across subfields. Stereological analysis revealed a pronounced loss of neurons within individual laminae of somatosensory cortex of affected mice and the novel finding of a loss of sensory relay thalamic neurons. These affected mice also exhibited profound astrocytic reactions that were most pronounced in the neocortex and thalamus, but diminished in other brain regions. These data provide the first direct evidence for neurodegenerative and reactive changes in the thalamocortical system in JNCL and emphasize the localized nature of these events. D 2005 Elsevier Inc. All rights reserved. Keywords: Batten disease; Juvenile neuronal ceroid lipofuscinosis; JNCL; CLN3; Thalamocortical degeneration; Astrocytosis; Lysosomal storage disorder Introduction The neuronal ceroid lipofuscinoses (NCLs) are the most common group of inherited neurodegenerative disorders of childhood with a collective incidence of up to 1 in 12,500 live births (Cooper, 2003; Hofmann and Peltonen, 2001). These fatal lysosomal disorders are characterized by blindness, marked * Corresponding author. Pediatric Storage Disorders Laboratory, Department of Neuroscience, Box P040, MRC Social Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, King’s College London, De Crespigny Park, London SE5 8AF, UK. Fax: +44 20 7848 0273. E-mail address: [email protected] (J.D. Cooper). Available online on ScienceDirect (www.sciencedirect.com). 0969-9961/$ - see front matter D 2005 Elsevier Inc. All rights reserved. doi:10.1016/j.nbd.2005.05.018 psychomotor deterioration and uncontrollable seizures (Hofmann and Peltonen, 2001; Gardiner, 2002). Juvenile NCL (JNCL) is the result of a mutation in the CLN3 gene, located in the p12.1 region of chromosome 16 (International Batten Disease Consortium, 1995). This most frequently occurring form of NCL has an age of onset between 4 and 10 years and typically results in premature death before the age of 30 (Gardiner, 2002). The Cln3 protein is predicted to be a highly hydrophobic 438 amino acid protein, containing multiple transmembrane domains (Mao et al., 2003). Although very little is known about the normal function of Cln3, colocalization studies have shown this protein to be expressed in the lysosome, endosome and synaptosomes (Haskell et al.; 2000, Jarvela et al., 1999). The most significant advances in understanding Cln3 function come from yeast that bear null mutations in Btn1p, the yeast ortholog of Cln3 (Pearce et al., 1999). The deletion of BTN1 results in a reduced vacuolar pH during early growth (Pearce et al., 1999), and influences the transport of basic amino acids into the vacuole (Kim et al., 2003), suggesting that Btn1p may play a role in pH homeostasis. Detailed quantitative information about neuropathological changes in JNCL is limited with qualitative studies restricted to autopsy material (Braak and Goebel, 1978, 1979). The loss of cortical neuron populations in JNCL has been well documented (Braak and Goebel, 1978, 1979; Haltia, 2003), together with selective neuronal loss and glial activation within the hippocampal formation (Haltia et al., 2001; Tyynelä et al., 2004). Since human autopsy material is not widely available, Cln3 null mutant mice (Cln3 / ) have been generated to enable the study of JNCL pathogenesis (Katz et al., 1999; Mitchison et al., 1999). These animals exhibit a JNCL-like phenotype and display widespread intracellular accumulation of autofluorescent pigment and a selective loss of GABAergic interneurons (Mitchison et al., 1999; Katz et al., 1999; Pontikis et al., 2004), together with an altered threshold for seizure generation (Kriscenski-Perry et al., 2002). A distinctive feature of Cln3 / mice is the early occurrence of reactive changes in both astrocytic and microglial 824 C.C. Pontikis et al. / Neurobiology of Disease 20 (2005) 823 – 836 cell populations, events which occur many months before widespread neuronal loss is evident (Pontikis et al., 2004). Cln3 / mice and individuals with JNCL also raise autoantibodies to glutamic acid decarboxylase (GAD65) that inhibit the activity of this enzyme, resulting in elevated levels of glutamate (Chattopadhyay et al., 2002). More recently, Cln3 knock-in mice (Cln3 Dex7/8 ) have been generated which accurately reproduce the 1.02-kb deletion in the Cln3 gene that is seen in over 85% of JNCL alleles (International Batten Disease Consortium, 1995), and also exhibit an NCL-like phenotype (Cotman et al., 2002). This phenotype may be more aggressive than that seen in Cln3 / mice, with a progressive neurologic disease and premature death of homozygous Cln3 Dex7/8 mice from 7 months onwards (Cotman et al., 2002). To further characterize the CNS of homozygous Cln3 Dex7/8 mice, we examined neurodegenerative and reactive phenotypes that are present in other mouse models of NCL, including Cln3 / mice (Mitchison et al., 1999; Pontikis et al., 2004). These models display a range of effects upon cortical thinning and neuronal loss between sensory and motor cortex (Bible et al., 2004; Cooper et al., 1999; Mitchison et al., 1999; Pontikis et al., 2004). Our analysis of these regions in homozygous Cln3 Dex7/8 mice also revealed different degrees of cortical thinning and selective effects upon cortical neuron number, together with significant loss of afferent thalamic neurons. These neuronal changes were accompanied by graded microglial activation and pronounced hypertrophy, but not hyperplasia, of astrocytes. These reactive changes also exhibited marked regional specificity, being most profound in the neocortex and within restricted nuclei of the thalamus. Taken together with recent findings from human JNCL (Tyynelä et al., 2004), these data further highlight the localized nature of reactive and neurodegenerative phenotypes in this disorder. Brains were subsequently removed and weighed before overnight post-fixation and cryoprotection at 4-C in a solution containing 30% sucrose in Tris-buffered saline (TBS: 50 mM Tris, pH 7.6) with 0.05% NaN3. Brains were subsequently processed and sectioned as described previously (Bible et al., 2004; Pontikis et al., 2004), with serial 40 Am frozen coronal sections stored at 40-C in cryoprotectant solution (TBS/30% ethylene glycol/15% sucrose/0.05% sodium azide) prior to histological processing. Nissl staining Materials and methods To provide direct visualization of neuronal morphology every sixth section through the CNS was stained with the Nissl dye cresyl violet as previously described (Bible et al., 2004; Pontikis et al., 2004). Briefly, sections were mounted onto gelatin-chrome alum-coated Superfrost microscope slides (VWR, Dorset, UK), air dried overnight and incubated for 20 min at 55-C in 0.05% cresyl fast violet and 0.05% acetic acid (VWR, Dorset, UK), rinsed in distilled water and differentiated through a graded series of alcohol before clearing in xylene (VWR, Dorset, UK) and coverslipping with DPX (VWR, Dorset, UK). These Nissl-stained sections were subsequently used for stereological analysis of regional volume, cortical thinning, cortical and thalamic neuron number, as described previously (Bible et al., 2004; Pontikis et al., 2004). To visualize autofluorescent storage material, selected sections from each animal were mounted upon Superfrost microscope slides (VWR, Dorset, UK) and immediately coverslipped with Vectashield aqueous mounting medium (Vector Laboratories, Peterborough, UK) (Bible et al., 2004; Griffey et al., 2003). Sections were viewed under conventional epifluorescence illumination on a Zeiss Axioskop2 MOT microscope (Carl Zeiss Ltd., Welwyn Garden City, UK) and images recorded at multiple wavelengths using a Zeiss Axiocam and Axiovision software (Carl Zeiss Ltd., Welwyn Garden City, UK). Animals Immunohistochemistry for interneuron markers Homozygous Cln3 Dex7/8 knock-in mice and control (+/+) mice were generated on a mixed 129Sv/Ev/CD1 strain background at the Molecular Neurogenetics Unit, Massachusetts General Hospital, Charlestown, MA, as described previously (Cotman et al., 2002). All mice used in this study were analyzed at 12 months of age and were littermates produced by crossing heterozygous Cln3 Dex7/8 knock-in mice that were from an F4 CD1 backcross generation. Mice of both sexes were used for this analysis since we have previously observed no significant difference in JNCL-like phenotype between male and female Cln3 Dex7/8 mice (Cotman et al., 2002). All perfusion procedures were carried out in accordance with NIH guidelines and under the animal care committee regulations at Massachusetts General Hospital. To survey representative interneuron populations in homozygous Cln3 Dex7/8 mice, adjacent one-in-six series of sections were immunohistochemically stained for the neuropeptide somatostatin (SOM) or the calcium binding protein parvalbumin (PV), as previously described (Bible et al., 2004; Pontikis et al., 2004). Sections were incubated for 15 min in 1% H2O2 in TBS, rinsed in TBS and blocked for 40 min in TBS/0.3% Triton X-100/15% normal goat serum (NGS) before overnight incubation at 4-C in the following polyclonal primary antisera (rabbit anti-SOM, Peninsula, CA, USA, 1:2000; rabbit anti-PV, Swant, Bellinzona, Switzerland, 1:5000) diluted in TBS with 10% normal goat serum and 0.3% Triton X-100. Sections were then rinsed in TBS and incubated for 2 h with secondary antiserum (biotinylated goat anti-rabbit IgG, Vector Laboratories, UK, 1:1000) in TBS/0.3% Triton X-100/10% NGS. Following rinsing in TBS, sections were incubated for 2 h in an avidin – biotin – peroxidase complex in TBS (Vectastain Elite ABC kit, Vector Laboratories, Peterborough, UK). Sections were next rinsed in TBS and immunoreactivity was visualized by incubation in 0.05% DAB (Sigma, Dorset, UK) and 0.001% H202 in TBS for 10 min (a time which represents saturation for this reaction). Sections were then transferred to excess ice-cold TBS and were rinsed again, mounted, air dried, cleared in xylene and coverslipped with DPX (VWR, Dorset, UK). Histological processing For histological analysis, 12-month-old homozygous Cln3 Dex7/8 knock-in mice and age-matched control littermates (n = 5) were fixed by transcardial perfusion. Mice were first deeply anaesthetized with sodium pentobarbitone (100 mg/kg) and transcardially perfused with vascular rinse (0.8% NaCl in 100 mM NaHPO4) followed by a freshly made and filtered solution of 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.4. C.C. Pontikis et al. / Neurobiology of Disease 20 (2005) 823 – 836 825 Immunohistochemistry for glial markers Measurements of total neuronal number and volume To assess the extent of glial activation in 12-month-old homozygous Cln3 Dex7/8 knock-in mice, adjacent one-in-six series of free-floating frozen sections were immunohistochemically stained using the standard immunoperoxidase protocol described above for detection of astrocytic (GFAP, S100h) and microglial (F4/80) markers using the following polyclonal primary antisera (polyclonal rabbit anti-cow GFAP, DAKO, Cambridge, UK, 1:1000; polyclonal goat anti-rabbit S100h, DAKO, Cambridge, UK, 1:1000; monoclonal rat anti-mouse F4/80, Serotec, Oxford, UK, 1:100). Sections were then rinsed in TBS with subsequent incubation in secondary anti-serum (swine anti-rabbit [GFAP and S100h], Vector Laboratories, Peterborough, UK, 1:400; and mouse adsorbed rabbit anti-rat [F4/80], Vector Laboratories, Peterborough, UK, 1:1000) followed by avidin – biotin – peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories, Peterborough, UK). Immunoreactivity was visualized by a standard DAB reaction and sections were mounted onto slides, air dried, cleared in xylene and coverslipped with DPX (VWR, Dorset, UK). To examine neuronal number and volume within individual cortical laminae, we used StereoInvestigator software to obtain unbiased optical fractionator estimates of neuronal number and nucleator estimates of neuronal volume from Nissl-stained sections. These estimates were obtained for neurons in lamina V of S1BF and M1 and of neurons in laminae II, III and IV in S1BF, together with afferent thalamic neurons in the ventral posterior nucleus (VPM/VPL). These measures were performed as described previously (Bible et al., 2004), with a random starting section chosen, followed by every sixth Nissl-stained section thereafter. The boundaries of M1 and S1BF regions and each individual lamina were defined by reference to landmarks in Paxinos and Franklin (2001). Only neurons with a clearly identifiable nucleus were sampled and all counts were carried out using a 100 oil objective (NA 1.4). The following sampling scheme was applied to the regions of interest. Grid area for lamina V M1 and S1BF 122,500 Am2, frame area 2141.3 Am2; grid area for S1BF laminae II and III of 122,500 Am2, frame area 2141.3 Am2; grid area for S1BF lamina IV of 40,000 Am2, frame area 1095.82 Am2; grid area for VPM/VPL of 122,500 Am2, frame area 3382.55 Am2. Measurements of volume, cortical and laminar thickness Measurements of interneuron number Unbiased Cavalieri estimates of the volume of the cortex, hippocampus, striatum, thalamus, hypothalamus and cerebellum were made from each animal, as described previously (Bible et al., 2004; Pontikis et al., 2004), with no prior knowledge of genotype. A sampling grid with appropriate spacing (500 Am cortex and cerebellum; 200 Am hippocampus; 300 Am striatum; 200 Am thalamus and hypothalamus; 400 Am cerebellum) was superimposed over Nissl-stained sections and the number of points covering the relevant areas counted using 2.5 objective. Regional volumes were expressed in Am3 and the mean volume of each region calculated for control and homozygous Cln3 Dex7/8 mice. Cortical thickness measurements were made on the same onein-six series of Nissl-stained sections for primary motor (M1), primary somatosensory (S1BF), limbic entorhinal (Lent) and primary visual cortex (V1) as defined by Paxinos and Franklin (2001). The length of perpendicular lines extending from the white matter to the pial surface was measured by placing 10 evenly spaced lines on each of three consecutive sections spanning each cortical region. These measurements were obtained blind to genotype using a 5 objective, exactly as described previously (Bible et al., 2004; Pontikis et al., 2004). Results were expressed as the mean cortical thickness in Am per region for control and homozygous Cln3 Dex7/8 animals. Individual laminar thickness measurements were also performed in M1, S1BF, Lent and V1 using the same Nissl-stained sections. As with cortical thickness measures, the thickness of each individual lamina was measured via a series of 10 perpendicular lines drawn in each of three consecutive sections using a 10 objective. Results were expressed as the mean laminar thickness in Am per region for control and homozygous Cln3 Dex7/8 animals. All volume and thickness analyses were carried out using StereoInvestigator software (Microbrightfield Inc., Williston, VT) on a Zeiss Axioskop2 MOT microscope (Carl Zeiss Ltd., Welwyn Garden City, UK) linked to a DAGEMTI CCD-100 camera (DAGE-MTI Inc., Michigan City, IN, USA). The number of GABAergic interneurons expressing SOM and PV in M1 and S1BF was determined using the design-based optical fractionator method (West et al., 1991). Immunoreactive neurons were sampled using a series of counting frames distributed over a grid and superimposed onto the section using StereoInvestigator software. The rostral and caudal limits of M1 and S1BF regions were defined according to Paxinos and Franklin (2001). The lateral to medial extent of M1 and S1BF were identified by comparison with an adjacent series of Nissl-stained sections and anatomical reference points. A 40 oil objective (NA 1.30) was then used to count clearly identifiable immunoreactive neurons, which fell within the dissector frame. The following sampling scheme was applied to the regions of interest; Grid area for SOM and PV in M1 and S1BF 38,275 Am2, frame area 20,191 Am2. Due to the comparatively low abundance of interneurons present in the hippocampus versus the neocortex, stereological methods prove inefficient at estimating hippocampal interneuron numbers without sampling the entire tissue (Bible et al., 2004). Instead, counts of the number of interneurons expressing SOM or PV were made, as described previously (Bible et al., 2004; Cooper et al., 1999; Pontikis et al., 2004) in defined hippocampal subfields. Counts were carried out under a 20 objective and only positively stained cells with clear neuronal morphology were counted. The number of interneurons in each hippocampal subfield was expressed as the mean number of neurons per section. Quantitative analysis of glial phenotype The optical density of GFAP and F4/80 immunoreactivity was assessed using a semi-automated thresholding image analysis system (Optimas, Media Cybernetics, Silver Springs, MD). This analysis was performed blind to genotype, as previously described (Bible et al., 2004; Pontikis et al., 2004). Forty non-overlapping images were captured, on three consecutive sections, through the cortical regions M1, S1BF and V1, striatum, hippocampal dentate gyrus and a combined measurement for the stratum oriens and 826 C.C. Pontikis et al. / Neurobiology of Disease 20 (2005) 823 – 836 CA1. All RGB images were captured via a live video camera (JVC, 3CCD, KY-F55B), mounted onto a Zeiss Axioplan microscope using a 40 objective and saved as JPEGs. All parameters including lamp intensity, video camera setup and calibration were maintained constant throughout image capturing. Images were subsequently analyzed using OPTIMAS 6.2 image analysis software (Media Cybernetics, Silver Springs, MD), using an appropriate threshold that selected the foreground immunoreactivity above background. This threshold was then applied as a constant to all subsequent images analyzed per batch of animals and reagent used to determine the specific area of immunoreactivity for each antigen in each region. Each field measured 120 Am wide, with a height of 90 Am. Therefore, the total area compiled from 40 fields in each region corresponded to 432,000 Am2. Macros were recorded to transfer the data to a Microsoft Excel spreadsheet and were subsequently analyzed statistically. Data were plotted graphically as the mean percentage area of immunoreactivity per field T SEM for each region. Measurements of astrocytic number To quantify changes in astrocyte number we obtained designbased optical fractionator estimates of S100h-positive soma, together with nucleator estimates of astrocyte volume. Immunoreactive astrocytes were sampled in S1BF and CA1/stratum oriens using a series of counting frames distributed over a grid and superimposed onto the section using StereoInvestigator software, exactly as described previously (Bible et al., 2004). The rostral and caudal limits of S1BF and CA1/stratum oriens regions were defined as described above. A 40 oil objective (NA 1.30) was then used to count clearly identifiable immunoreactive astrocytes, which fell within the dissector frame. The following sampling scheme was applied to the regions of interest; Grid area for S100h in S1BF and CA1/oriens 20,191 Am2, frame area 122,500 Am2. Statistical analysis The statistical significance of differences between genotypes of all quantitative data was assessed using a one-way ANOVA (SPSS 11.5 software, SPSS Inc, Chicago, IL), with statistical significance considered at P < 0.05. The mean coefficient of error (CE) for all individual optical fractionator and nucleator estimates was calculated according to the method of Gundersen and Jensen (1987) and was less than 0.08 in all these analyses. Results Homozygous Cln3 Dex7/8 mice exhibit widespread accumulation of autofluorescent storage material Macroscopic examination of the brains of 12-month-old Cln3 Dex7/8 homozygotes did not reveal an obvious degenerative phenotype, with the cortex and cerebellum of affected mice appearing indistinguishable from controls (data not shown). Consistent with these observations, the brains of homozygous Cln3 Dex7/8 mice were lighter than age-matched controls, but this reduction did not reach statistical significance (control 494 T 43 mg; Cln3 Dex7/8 456 T 39 mg, P = 0.128, n = 5). Microscopically, homozygous Cln3 Dex7/8 mice exhibited widespread intracellular accumulation of autofluorescent storage material within neuronal soma throughout the CNS (Fig. 1). In mutant mice, this storage material was present as punctate material that fluoresced at multiple wavelengths, as viewed by conventional epifluorescence microscopy (Figs. 1B – D, F – G). In Fig. 1. Accumulation of autofluorescent storage material in homozygous Cln3 Dex7/8 mice. (A – H) Representative images of unstained coronal sections through the primary motor cortex (A – D) and cerebellum (E – H) viewed by epifluorescence using rhodamine (Rho) or FITC filter sets. Sections from 12-month-old homozygous Cln3 Dex7/8 mice display widespread intracellular accumulation of storage material (B – D, F – H) that fluoresces with both filter sets and appears yellow in merged images (D, H). In contrast, sections from littermate controls display a low level of background tissue fluorescence and many fewer scattered deposits of storage material within the cortex (A), and a low level of autofluorescence within Purkinje neurons of the cerebellum (E). C.C. Pontikis et al. / Neurobiology of Disease 20 (2005) 823 – 836 contrast, control littermates displayed a low level of background autofluorescence and an age-related and sparsely scattered accumulation of autofluorescent material (Fig. 1A) that was more pronounced in Purkinje neurons of the cerebellum (Fig. 1E). Lack of significant regional atrophy in homozygous Cln3 Dex7/8 knock-in mice Since homozygous Cln3 Dex7/8 brains did not display an overt phenotype macroscopically, we carried out a stereological survey of regional volume and cortical thickness to look for more subtle neurodegenerative changes in Nissl-stained sections. Cavalieri estimates of regional volume revealed that although many CNS regions including the cortex, hippocampus and cerebellum were reduced in size in Cln3 Dex7/8 mice, the thalamus was the only structure that displayed significantly reduced volume in homozygous Cln3 Dex7/8 mice (Fig. 2). Regional effects upon cortical thinning and lamination in homozygous Cln3 Dex7/8 mice Because measurements of overall cortical volume cannot discriminate between events in different cortical subfields, we carried out a series of cortical thickness measurements of primary motor (M1), somatosensory barrel field (S1BF), primary visual (V1) and lateral entorhinal (Lent) regions (Fig. 3). Homozygous Cln3 Dex7/8 mice showed variable effects upon cortical thickness in different regions. There was significant thinning of S1BF and Lent cortex, but a significant increase in the thickness of M1 (Fig. 3A). In contrast, V1 exhibited less pronounced thinning in homozygous Cln3 Dex7/8 mice that was not significant versus littermate controls (Fig. 3A). To determine whether these changes were due to laminarspecific events, we made measurements of individual lamina thickness (Figs. 3B – E). Although significant reductions in the thickness of lamina I were consistently seen in S1BF, V1 and Lent and the thickness of laminae II + III in M1, S1BF and Lent (Figs. 3B – D), the remaining laminae in these cortical regions displayed a 827 complex series of contrasting changes in thickness (Table 1). The most extreme example was in Lent which displayed significant reductions in the thickness of laminae I and II, but significantly increased thickness of laminae III and IV (Fig. 3D). The increase in the thickness of M1 in homozygous Cln3 Dex7/8 mice appeared to be largely due to a significant increase in the thickness of lamina V (Fig. 3E), whereas all other laminae were either unchanged (I and VI) or displayed a small, but significant thinning (II + III). Regional and laminar effects upon neuronal number in homozygous Cln3 Dex7/8 mice To investigate whether these changes in individual laminar thickness in homozygous Cln3 Dex7/8 mice were the result of effects upon alterations in neuronal number and/or neuronal size, we obtained optical fractionator estimates of neuronal number and nucleator estimates of neuronal volume in Nissl-stained sections. These data were collected for lamina V pyramidal neurons in S1BF and M1, two cortical regions which displayed contrasting changes in the thickness of this lamina, in addition to lamina IV granule neurons of S1BF and a combined measure of laminae II and III neurons in S1BF (Fig. 4). Although there was a trend to reduced neuronal number, lamina V pyramidal neurons were not significantly lost in either M1 or S1BF of homozygous Cln3 Dex7/8 mice (Fig. 4A). In contrast, there was a significant loss of neurons in laminae II and III of S1BF of mutant mice, together with a significant loss of granule neurons in lamina IV of this cortical region (Fig. 4A). Nucleator estimates of neuronal volume revealed only minor changes in cell size in individual laminae (Fig. 4B), none of which reached statistical significance. To determine the effect of this loss of granule neurons in S1BF upon afferent thalamic neurons, we obtained unbiased stereological estimates of neuronal number and volume in the ventral posterior thalamic nucleus (VPM/VPL). These analyses revealed a significant loss of neurons within VPM/VPL of homozygous Cln3 Dex7/8 mice (Fig. 4A), although these neurons were not significantly hypertrophied (Fig. 4B). Fig. 2. Absence of significant regional atrophy in homozygous Cln3 Dex7/8 mice. Unbiased Cavalieri estimates of regional volume reveal no significant regional atrophy in Cln3 Dex7/8 homozygotes versus littermate controls (+/+) at 12 months of age, with the exception of the thalamus (Thal). Regions examined include cortical mantle (Cortex); hippocampus (Hipp); hypothalamus (Hypoth); striatum (CPu); whole cerebellum (Cb total); cerebellar vermis (Cb vermis); and lateral hemisphere (Cb lat). *P < 0.05, one-way ANOVA. 828 C.C. Pontikis et al. / Neurobiology of Disease 20 (2005) 823 – 836 Fig. 3. Subregion- and laminar-specific changes in cortical thickness in homozygous Cln3 Dex7/8 mice. (A) Cortical thickness measurements. Compared with littermate controls (+/+), thinning of the cortical mantle in Cln3 Dex7/8 homozygotes was more pronounced in primary somatosensory barrel field (S1BF) and entorhinal (Lent) cortex than primary visual (V1), or primary motor (M1) cortex which exhibited a small, but significant increase in overall thickness. (B – E) Laminar thickness measurements in these cortical regions reveal a complex series of changes in thickness of individual cortical laminae in 12-month-old homozygous Cln3 Dex7/8 mice compared with littermate controls (+/+). *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA. These data are also summarized in Table 1. Survival of GABAergic interneurons in homozygous Cln3 Dex7/8 Loss of GABAergic interneurons is a common feature of other murine models of NCL (Cooper, 2003; Mitchison et al., 2004). To determine whether these neuronal populations were also affected in homozygous Cln3 Dex7/8 mice, the expression of parvalbumin (PV) and somatostatin (SOM) were surveyed in the cortex (Fig. 5A) and hippocampus (Figs. 5B and C). These markers are usually expressed in representative interneuron populations (Freund and Buzsáki, 1996), which are consistently affected in mouse models of infantile NCL (Bible et al., 2004; Jalanko et al., 2005), juvenile NCL (Mitchison et al., 1999; Pontikis et al., 2004) and variant late infantile NCL (Cooper et al., 1999; Kopra et al., 2004). Primary motor and somatosensory cortex Optical fractionator estimates revealed a general trend to a reduced number of interneurons immunoreactive for PV and SOM in M1 and S1BF, but this loss of interneurons did not reach significance for interneurons stained for either antigen in these cortical regions (Fig. 5A). Hippocampus We next examined interneuron populations that are immunoreactive for these markers in the hippocampal formation of homozygous Cln3 Dex7/8 mice. There was an overall trend to reduced number of SOM-positive interneurons in homozygous Cln3 Dex7/8 mice, although this did not reach significance in any hippocampal subfield (Fig. 5C). Effects upon the number of PV- C.C. Pontikis et al. / Neurobiology of Disease 20 (2005) 823 – 836 829 Table 1 Tabular depiction of the significant changes in individual laminar thickness in the primary motor (M1), somatosensory barrel field (S1BF), primary visual (V1) and entorhinal cortex (Lent) of 12-month-old Cln3 Dex7/8 compared with age-matched controls (+/+) M1 S1BF V1 Lent I II/III III IV V VI Total thickness NS , , , , , NS , na na na j na j , j j , NS NS NS , j NS j , NS , Significantly j = thicker in Cln3 Dex7/8 ; , = significantly thinner in Cln3 Dex7/8 ; NS = not significant; na = not applicable, M1 has no lamina IV. A combined measurement of laminae II and III was made in all regions except Lent where these laminae were measured separately. positive interneurons were more variable between hippocampal subfields in homozygous Cln3 Dex7/8 mice, but none of these changes reached statistical significance (Fig. 5B). Fig. 4. Thalamocortical neuron loss in homozygous Cln3 Dex7/8 mice. (A) Optical fractionator estimates of neuronal number revealed the significant loss of neurons in laminae II and III and lamina IV granule neurons, but no significant loss of lamina V pyramidal neurons in primary somatosensory barrelfield cortex (S1BF) or primary motor cortex (M1) of homozygous Cln3 Dex7/8 mice compared with littermate controls (+/+). There was also a significant reduction in the number of neurons in the ventral posterior thalamic nucleus (VPM/VPL) of mutant mice. (B) Nucleator estimates of neuronal volume revealed no significant atrophy or hypertrophy in any laminae of Cln3 Dex7/8 homozygotes. *P < 0.05, ***P < 0.001, one-way ANOVA. Fig. 5. Interneuron populations are not significantly affected in homozygous Cln3 Dex7/8 mice. (A) Persistence of cortical interneurons in aged Cln3 Dex7/8 homozygotes. Optical fractionator estimates of parvalbumin (PV) and somatostatin (SOM)-positive interneuron number in the primary motor (M1) and somatosensory barrelfield (S1BF) cortex reveal no significant loss of these neurons in 12-month-old Cln3 Dex7/8 homozygotes compared with littermate controls (+/+). (B – C) Survival of hippocampal interneurons in aged homozygous Cln3 Dex7/8 mice. Counts of hippocampal interneuron number revealed no significant loss of interneurons immunoreactive for either parvalbumin (B) or somatostatin (C) in any subfield of 12month-old Cln3 Dex7/8 and age-matched controls (+/+). Regionally restricted astrocytic and microglial activation in homozygous Cln3 Dex7/8 mice To examine glial responses in homozygous Cln3 Dex7/8 mice, we first surveyed the distribution of cells immunoreactive for the astrocytic markers GFAP and S100h, in addition to the microglial marker F4/80. Subsequently, we used image analysis to quantify the expression of these markers in the striatum, cortical subregions 830 C.C. Pontikis et al. / Neurobiology of Disease 20 (2005) 823 – 836 M1 and S1BF; and hippocampal subfields hilus, CA1, CA2 and CA3 using standard methods (Bible et al., 2004; Pontikis et al., 2004). Reactive astrocytosis may represent hypertrophy of astrocytes (thickening of processes and changes in cell body volume) which may be independent of cell proliferation. Therefore, we also obtained optical fractionator estimates of the number of astrocytes immunoreactive for S100h, a calcium binding protein that is expressed predominantly in astrocytes (Boyes et al., 1986). GFAP- and S100b-positive astrocytes In the neocortical grey matter of control mice, only few GFAPpositive astrocytes were present, predominantly in lamina VI and in lamina I adjacent to the pial surface (Fig. 6A). In marked contrast, homozygous Cln3 Dex7/8 mice displayed a profound astrocytosis across the cortical mantle with intensely GFAP-immunoreactive astrocytes present in both superficial and deeper cortical laminae (Fig. 6B), with positive astrocytes extending in clusters across all laminae. At higher power, these GFAP-positive astrocytes displayed marked hypertrophy with numerous thickened and branched processes and enlarged soma in homozygous Cln3 Dex7/8 mice compared to control littermates (Figs. 6A and B). In marked contrast to GFAP, S100h immunoreactivity within the neocortex was present within numerous intensely stained cell somas that were distributed evenly across laminae and did not differ obviously between animals of either genotype (Figs. 6C and D). Subcortically, this astrocytic response was far less pronounced with reduced staining for GFAP present within the hippocampus and cerebellum (data not shown). In contrast, the thalamus of homozygous Cln3 Dex7/8 mice exhibited pronounced GFAP immunoreactivity compared to controls (Figs. 6E and F). However, this staining for GFAP was not uniformly distributed but was highest in individual thalamic nuclei, most notably within the ventral posterior (VPM/VPL), lateral regions of the laterodorsal (LD) Fig. 6. Prominent astrocytosis in homozygous Cln3 Dex7/8 mice. (A, B) Immunohistochemical staining for GFAP reveals profound astrocytosis in the cortex of 12-month-old Cln3 Dex7/8 homozygotes (B) compared with age-matched controls (+/+, A). Within the primary motor cortex (M1) many hypertrophic GFAPpositive astrocytes bearing numerous thickened processes were present in both deep and superficial laminae of mutant mice (B). In contrast, there were no obvious differences in the distribution or number of S100h-positive astrocytes in M1 of animals of either genotype (C, D). Compared to controls (E), the thalamus of Cln3 Dex7/8 homozygotes exhibited pronounced astrocytosis that was confined to individual thalamic nuclei (F), with GFAP-positive astrocytes prominent in the ventral posterior (VPM/VPL), mediodorsal (MD) and lateral regions of the laterodorsal (LD) thalamic nuclei, but virtually absent in the adjacent ventromedial (VM), posterior (Po) and centrolateral (CL) thalamic nuclei. C.C. Pontikis et al. / Neurobiology of Disease 20 (2005) 823 – 836 and the mediodorsal (MD) thalamic nuclei, but virtually absent in the adjacent ventromedial (VM), posterior (Po) or intralaminar thalamic nuclei (Fig. 6F). Quantitative analysis of astrocytosis Consistent with these morphologic observations, quantitative image analysis revealed a significant and widespread increase in 831 GFAP expression in both superficial and deeper cortical layers (Fig. 7A). This upregulation was not confined to any cortical region with similar increases in GFAP staining in M1, S1BF, V1 and Lent (Fig. 7A). In contrast to these events in the neocortex, quantitative analysis revealed a range of different astrocyte responses in the hippocampus (Fig. 7B), cerebellum (Fig. 7C) and subcortical structures of Cln3 Dex7/8 homozygotes. Unexpectedly, the hippocampal dentate Fig. 7. Regionally variable astrocytosis in homozygous Cln3 Dex7/8 mice. (A) Quantitative image analysis reveals the widespread and significantly increased expression of GFAP in the superficial and deep cortical laminae of Cln3 Dex7/8 homozygotes compared with littermate controls (+/+) at 12 months of age. (B – C) In contrast, hippocampal (B) and cerebellar grey matter (C) display significantly reduced levels of GFAP in mutant mice compared with controls. Areas surveyed include striatum (CPu), hippocampal dentate gyrus (DG), hippocampal stratum oriens and CA1 (Oriens CA1), cerebellar white matter (WM) and molecular and granule cell layers (Mol + Gr) in the lateral hemispheres (Lat) and vermis (Verm). (D) Optical fractionator estimates revealed no significant change in the number of S100h-positive astrocytes in either the primary somatosensory barrel field cortex (S1BF) or hippocampal CA1 and stratum oriens of homozygous Cln3 Dex7/8 mice, compared with littermate controls (+/+). ***P < 0.001, one-way ANOVA. 832 C.C. Pontikis et al. / Neurobiology of Disease 20 (2005) 823 – 836 gyrus, stratum oriens and CA1 of homozygous Cln3 Dex7/8 mice exhibited significantly reduced GFAP expression compared to control littermates (Fig. 7B). GFAP expression in homozygous Cln3 Dex7/8 mice was similarly reduced in the grey matter of cerebellar lateral hemisphere and vermis, but was unchanged in the white matter within these regions of the cerebellum (Fig. 7C). To determine whether these changes in GFAP expression reflected changes in the number of astrocytes, we next obtained unbiased stereological estimates of the number of S100h-positive astrocytes. These optical fractionator data were collected within one representative region that displayed increased GFAP expression (S1BF) and one that displayed decreased GFAP expression (stratum oriens and CA1 of the hippocampus) but did not reveal any significant change in astrocyte number within either region of homozygous Cln3 Dex7/8 mice compared with control littermates (Fig. 7D). F4/80-positive microglia Compared to control littermates, F4/80 immunoreactive microglia were consistently more prominent in homozygous Cln3 Dex7/8 mice, with these differences again more prominent in the neocortex than other CNS regions (Fig. 8). In the neocortex of control littermates, the widespread distribution of microglia was revealed by pale F4/80 immunoreactivity within cell soma and thin processes extending into the neuropil (Figs. 8A and C). In contrast, throughout the cortical mantle of Cln3 Dex7/8 homozygotes, there were intensely F4/80 immunoreactive microglia with numerous ramified processes, often with more prominent cell soma (Figs. 8B and D). In homozygous Cln3 Dex7/8 mice, these F4/ 80 immunoreactive microglia were present with no particular focus and across all laminae, although F4/80-positive cells frequently displayed more morphological signs of activation in deeper laminae with fewer thickened processes. Fig. 8. Activation of microglia in homozygous Cln3 Dex7/8 mice. Immunohistochemical staining for F4/80 revealed graded activation of microglia in 12-monthold homozygous Cln3 Dex7/8 mice compared with littermate controls (+/+). At this age, F4/80-positive microglia with numerous ramified processes in the primary motor cortex (M1) were more darkly stained in Cln3 Dex7/8 homozygotes (B, D) than littermate controls (A, C). This difference between genotypes was less pronounced in the striatum (CPu), although numerous partially activated microglia with enlarged soma and short thickened processes were evident in homozygous Cln3 Dex7/8 mice (F) compared to the microglia with thin ramified processes that were present in littermate controls (E). C.C. Pontikis et al. / Neurobiology of Disease 20 (2005) 823 – 836 Differences in the relative intensity of F4/80 staining between genotypes were less marked in subcortical structures (Figs. 8E and F), although homozygous Cln3 Dex7/8 mice again displayed more morphological evidence of microglial activation in these regions. Although complete morphological transformation to brain macrophage-like morphology in homozygous Cln3 Dex7/8 mice was seldom seen, these mice frequently displayed F4/80 immunoreactive microglia with enlarged soma and numerous short thickened processes (Fig. 8F), compared to the threadlike and ramified processes in control mice (Fig. 8E). Discussion This study represents the first detailed description of the CNS of homozygous Cln3 Dex7/8 mice, a model that recapitulates the major deletion present in the vast majority of JNCL alleles (Cotman et al., 2002). Our data revealed no interneuron loss or significant regional atrophy in Cln3 Dex7/8 homozygotes by 12 months of age. The marked exception was the thalamus, which displayed regional atrophy and a significant loss of somatosensory relay neurons in mutant mice, together with loss of their target neurons within the somatosensory cortex. These findings provide significant new data of neuronal loss within the thalamocortical system in JNCL, which was accompanied by a pronounced astrocytic response within individual thalamic nuclei of Cln3 Dex7/8 homozygous mice. Astrocytic activation was prominent cortically, yet was diminished within the hippocampus and cerebellum of mutant mice. These data provide further evidence that both neuronal and glial responses in JNCL are localized and regionally specific, as typified by our novel findings within the thalamocortical system of homozygous Cln3 Dex7/8 mice. Homozygous Cln3 Dex7/8 mice as a model for JNCL Bearing the major deletion that is present in over 85% of CLN3 alleles in JNCL (International Batten Disease Consortium, 1995), Cln3 Dex7/8 knock-in mice are an accurate model of this disorder (Cotman et al., 2002). Certainly, the homozygous Cln3 Dex7/8 mice used in this study exhibit an early onset of neurologic deficits (Cotman et al., 2002) that are more pronounced than the relatively mild retinal and neurological phenotypes of Cln3 / (Katz et al., 1999; Mitchison et al., 1999; Seigel et al., 2002). Nevertheless, any comparisons between these mice are complicated by the different strain backgrounds these models have been raised on (Mitchison et al., 1999; Cotman et al., 2002). As such, it will be essential to reevaluate their phenotypes once these mouse models of JNCL are all available upon a common strain background. Selective loss of cortical neurons in homozygous Cln3 Dex7/8 mice Although cortical atrophy is a consistent feature of various mouse models of NCL, including Cln3 / (Mitchison et al., 1999; Pontikis et al., 2004), PPT1 / (Bible et al., 2004) and TPP1deficient mice (Sleat et al., 2004), atrophy of the neocortex of homozygous Cln3 Dex7/8 mice was relatively mild and may only become more pronounced with increased age. Indeed, since approximately 20% of these mice do not reach 12 months of age (Cotman et al., 2002), it is possible that by performing our analysis at this age, we have selected mice that may present with a less pronounced or delayed neurodegenerative phenotype. 833 Our data from 12-month-old homozygous Cln3 Dex7/8 knock-in mice did reveal a range of selective effects upon cortical thickness that varied between different subfields (Fig. 3). These effects extended to individual laminae and, as highlighted by data from the somatosensory cortex of homozygous Cln3 Dex7/8 mice, to neuronal number within these laminae (Fig. 4). The most pronounced loss within this cortical region was of laminae II and III neurons, which supply commissural and association projections to other areas of cortex. The loss of these neurons may potentially have significant consequences for coordinating neuronal activity between hemispheres and cortical regions. In this respect, it will be important to determine the functional correlates of these pathological changes in the cortex of homozygous Cln3 Dex7/8 mice. Although GABAergic interneuron subpopulations were depleted to different extents in various cortical and hippocampal subfields, unlike human JNCL (Tyynelä et al., 2004), the Cln3 / model of JNCL (Mitchison et al., 1999; Pontikis et al., 2004) or other mouse models of NCL (Cooper et al., 1999; Bible et al., 2004; Kopra et al., 2004; Jalanko et al., 2005), these cell populations were not significantly affected in homozygous Cln3 Dex7/8 mice. The lack of significant loss of these interneurons in Cln3 Dex7/8 homozygotes may be characteristic of these mice, or alternatively, this phenotype may take longer to develop. Loss of thalamic neurons in homozygous Cln3 Dex7/8 mice Our analysis of regional volume was informative revealing thalamic atrophy in homozygous Cln3 Dex7/8 mice, a phenotype that is also apparent in PPT1-deficient mice (Bible et al., 2004). Although the thalamus typically displays a reduced MRI signal intensity in symptomatic INCL and JNCL patients (Autti et al., 1997; Vanhanen et al., 2004), the cellular basis of this hypointensity has remained unknown. Indeed, in contrast to the wealth of information available for the cortex and hippocampus (Braak and Goebel, 1978, 1979; Haltia, 2003; Haltia et al., 2001; Tyynelä et al., 2004), neuropathological data of neuronal loss within subcortical structures in JNCL are extremely limited (Braak and Braak, 1987; Braak et al., 1979). As such, our data from homozygous Cln3 Dex7/8 mice are significant in providing the first direct evidence for the loss of thalamic relay neurons in this disorder. It is unclear whether thalamic neurons are themselves inherently vulnerable in JNCL, or degenerate in response to events within the cortex. Determining the precise sequence of events in the thalamocortical system during pathogenesis will be crucial to distinguish between these distinct mechanistic possibilities. The clinical consequences of these events within the thalamocortical system remains unclear, although progressive changes in somatosensory evoked potentials are consistently reported in multiple forms of NCL (Tackmann and Kuhlendahl, 1979; Vercruyssen et al., 1982; Vanhanen et al., 1997; Lauronen et al., 1997), including JNCL (Lauronen et al., 1997, 1999). Indeed, it has been suggested that progressive thalamic dysfunction may also contribute to the sleep disturbances that are characteristic of the NCLs (Vanhanen et al., 1997). It will be highly informative to determine whether the loss of thalamic neurons and/or their cortical targets contributes to similar phenotypes in homozygous Cln3 Dex7/8 knock-in mice. We have already reported motor coordination problems by 12 months of age in homozygous Cln3 Dex7/8 knock-in mice (Cotman et al., 2002), but this current study demonstrated no overt effects within the cerebellum of these mice. Although subtle effects on 834 C.C. Pontikis et al. / Neurobiology of Disease 20 (2005) 823 – 836 other neuronal populations may also be present, our data suggest that neurologic problems in homozygous Cln3 Dex7/8 knock-in mice may involve altered sensory feedback through the thalamus. In this context, it will be important to correlate sensory behaviors and other forms of neurological impairment with pathological changes in the thalamocortical system during disease progression. Prominent astrocytosis and graded microglial responses in Cln3 Dex7/8 homozygotes Pronounced gliosis is a consistent feature of autopsy material from individuals with JNCL (Braak and Goebel, 1978, 1979; Tyynelä et al., 2004) and other forms of NCL (Haltia et al., 1973a,b; Herva et al., 2000; Tyynelä et al., 1997, 2004). Similar reactive events are evident in mouse models of CLN8 (Cooper et al., 1999), and infantile NCL (Bible et al., 2004; Jalanko et al., 2005), but a considerably more subtle glial phenotype is evident in Cln3 / (Pontikis et al., 2004). Markers of astrocytic and microglial activation are significantly elevated by 5 months of age in Cln3 / , but this activation remains at a low level with no morphological transformation to brain macrophages or obvious astrocytic hypertrophy (Pontikis et al., 2004). Our data from Cln3 Dex7/8 homozygotes provide a distinctly different picture of reactive changes in these mice, with widespread astrocyte hypertrophy across the cortical mantle and variable responses subcortically (Fig. 6). Curiously, there was little evidence of astrocytic proliferation or hyperplasia in homozygous Cln3 Dex7/8 mice, with no difference in the number of S100hpositive astrocytes between animals of either genotype. These S100h data also suggest that reduced GFAP immunoreactivity evident in the cerebellum and hippocampus of homozygous Cln3 Dex7/8 mice appears to represent downregulation of this marker rather than the targeting of astrocytes that has been proposed in Cln3 / (Pontikis et al., 2004). Although astrocytes classically mediate responses to neuronal injury (Raivich et al., 1999), emerging evidence suggests these cell types play dynamic roles in regulating fluid homeostasis (Simard and Nedergaard, 2004) and controlling local concentrations of glutamate, influencing neuronal activity and synaptic efficacy (Oliet et al., 2001; Piet et al., 2002). Although the localized events that trigger astrocytosis in homozygous Cln3 Dex7/8 mice remain unclear, these may involve neuronal damage due to accumulated storage material. However, in contrast to the widespread accumulation of storage material (Fig. 1), astrocytic activation within the thalamus was restricted to individual nuclei (Fig. 6), with particularly prominent GFAP staining within the VPM/VPL which also exhibited neuronal loss (Fig. 4). These reactive changes may represent a localized protective response to the effects of the CLN3 mutation, as has been suggested in human JNCL (Tyynelä et al., 2004). In this respect, investigating the precise spatiotemporal relationship between astrocytosis and neuronal loss at different stages of disease progression is likely to be highly informative. In contrast to these prominent astrocytic responses in Cln3 Dex7/8 homozygotes, the microglial responses in these mice were markedly less pronounced. The activation of microglia is a progressive and graded phenomenon that takes place in different Fstages_ from extensively ramified cells to full blown brain macrophages (Raivich et al., 1999), and has been considered a sensitive marker of local neuronal damage (Streit, 2000, 2002). In Cln3 Dex7/8 homozygous mice this microglial activation rarely reached its full extent to reveal brain macrophage morphology, even in areas where a profound astrocytic response was evident. These data suggest that whatever underlying molecular cues trigger astrocytosis in Cln3 Dex7/8 knock-in mice, these are not sufficient to promote full morphological transformation of microglia. Alternatively, microglia may themselves be targeted by the effects of Cln3 mutation as has been suggested in Cln3 / mice (Pontikis et al., 2004). Taken together with data from other mouse models of NCL (Cooper et al., 1999; Lam et al., 1999; Mitchison et al., 1999; Bible et al., 2004), sheep with NCL (Oswald et al., 2001, in press) and human NCL (Tyynelä et al., 2004), our data suggest that highly regionalized interactions occur between neurons and glia at different stages of disease progression. Whether these events contribute directly to subsequent neurodegeneration has not been demonstrated, but the suggestion that neuron – glial interactions differ markedly between CNS regions may provide important clues to the pathogenesis of these disorders. Acknowledgments We would like to thank the other members of the PSDL for their valuable contributions; Noreen Alexander and Stephen Shemilt for their expert advice; Dr. Payam Rezaie for his continuing collaboration; and Drs. David Pearce, Alison Barnwell and Jill Weimer for constructive comments on the manuscript. These studies were supported by National Institutes of Health grants NS41930 (JDC), NS33648 (MEM), European Commission 6th Framework Research Grant LSHM-CT-2003-503051 (JDC), a post-doctoral fellowship to SLC from the Batten Disease Support and Research Association, and grants to JDC from The Natalie Fund, Batten Disease Family Association, Batten Disease Research Trust and the Remy Fund. References Autti, T., Raininko, R., Santavuori, P., Vanhanen, S.L., Poutanen, V.P., Haltia, M., 1997. MRI of neuronal ceroid lipofuscinosis: II. Postmortem MRI and histopathological study of the brain in 16 cases of neuronal ceroid lipofuscinosis of juvenile or late infantile type. Neuroradiology 39, 371 – 377. Bible, E., Gupta, P., Hofmann, S.L., Cooper, J.D., 2004. Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 (PPT1) null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol. Dis. 16, 346 – 359. Boyes, B.E., Kim, S.U., Lee, V., Sung, S.C., 1986. Immunohistochemical co-localization of S-100b and the glial fibrillary acidic protein in rat brain. Neuroscience 17, 857 – 865. Braak, H., Braak, E., 1987. Projection neurons of basolateral amygdaloid nuclei develop meganeurites in juvenile and adult human neuronal ceroid lipofuscinosis. Clin. Neuropathol. 6, 116 – 119. Braak, H., Goebel, H.H., 1978. Loss of pigment-laden stellate cells: a severe alteration of the isocortex in juvenile neuronal ceroid-lipofuscinosis. Acta Neuropathol. (Berl.) 42, 53 – 57. Braak, H., Goebel, H.H., 1979. Pigmentoarchitectonic pathology of the isocortex in juvenile neuronal ceroid-lipofuscinosis: axonal enlargements in layer IIIab and cell loss in layer V. Acta Neuropathol. (Berl.) 46, 79 – 83. Braak, H., Braak, E., Gullotta, F., Goebel, H.H., 1979. Pigment-filled appendages of the small spiny neurons: a severe pathological change of the striatum in neuronal ceroid lipofuscinosis. Neuropathol. Appl. Neurobiol. 5, 389 – 394. C.C. Pontikis et al. / Neurobiology of Disease 20 (2005) 823 – 836 Chattopadhyay, S., Ito, M., Cooper, J.D., Brooks, A.I., Curran, T.M., Powers, J.M., Pearce, D.A., 2002. An autoantibody inhibitory to glutamic acid decarboxylase in the neurodegenerative disorder Batten disease. Hum. Mol. Genet. 11, 1421 – 1431. Cooper, J.D., 2003. Progress towards understanding the neurobiology of Batten disease or neuronal ceroid lipofuscinosis. Curr. Opin. Neurol. 16, 121 – 128. Cooper, J.D., Messer, A., Feng, A.K., Chua-Couzens, J., Mobley, W.C., 1999. Apparent loss and hypertrophy of interneurons in a mouse model of neuronal ceroid lipofuscinosis: evidence for partial response to insulin-like growth factor-1 treatment. J. Neurosci. 19, 2556 – 2567. Cotman, S.L., Vrbanac, V., Lebel, L.A., Lee, R.L, Johnson, K.A., Donahue, L.R., Teed, A.M., Antonellis, K., Bronson, R.T., Lerner, T.J., MacDonald, M.E., 2002. Cln3 Dex7/8 knock-in mice with the common JNCL mutation exhibit progressive neurologic disease that begins before birth. Hum. Mol. Genet. 11, 2709 – 2721. Freund, T.F., Buzsáki, G., 1996. Interneurons of the hippocampus. Hippocampus 6, 347 – 470. Gardiner, R.M., 2002. Clinical features and molecular genetic basis of the neuronal ceroid lipofuscinoses. Adv. Neurol. 89, 211 – 215. Griffey, M., Bible, E., Vogler, C., Levy, B., Gupta, P., Cooper, J.D., Sands, M., 2003. Adeno-associated virus 2-mediated gene therapy decreases autofluorescent storage material and increases brain mass in a murine model of infantile neuronal ceroid lipofuscinosis (INCL). Neurobiol. Dis. 16, 360 – 369. Gundersen, H.J., Jensen, E.B., 1987. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 147, 229 – 263. Haltia, M., 2003. The neuronal ceroid-lipofuscinoses. J. Neuropathol. Exp. Neurol. 62, 1 – 13. Haltia, M., Rapola, J., Santavuori, P., 1973a. Infantile type of so-called neuronal ceroid-lipofuscinosis. Histological and electron microscopic studies. Acta Neuropathol. (Berl.) 26, 157 – 170. Haltia, M., Rapola, J., Santavuori, P., Keranen, A., 1973b. Infantile type of so-called neuronal ceroid-lipofuscinosis: II. Morphological and biochemical studies. J. Neurol. Sci. 18, 269 – 285. Haltia, M., Herva, R., Suopanki, J., Baumann, M., Tyynelä, J., 2001. Hippocampal lesions in the neuronal ceroid lipofuscinoses. Eur. J. Paediatr. Neurol. 5, 209 – 211 (Suppl.). Herva, R., Tyynelä, J., Hirvasniemi, A., Syrjakallio-Ylitalo, M., Haltia, M., 2000. Northern epilepsy: a novel form of neuronal ceroid-lipofuscinosis. Brain Pathol. 10, 215 – 222. Haskell, R.E., Carr, C.J., Pearce, D.A., Bennett, M.J., Davidson, B.L., 2000. Batten disease: evaluation of Cln3 mutations on protein localization and function. Hum. Mol. Genet. 9, 735 – 744. Hofmann, S.L., Peltonen, L., 2001. The neuronal ceroid lipofuscinosis. In: Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D. (Eds.), The Metabolic and Molecular Basis of Inherited Disease, vol. III, 8th ed. McGraw-Hill, New York, pp. 3877 – 3894. International Batten Disease Consortium, 1995. Isolation of a novel gene underlying Batten disease. Cell 82, 949 – 957. Jalanko, A., Vesa, J., Manninen, T., von Schantz, C., Minye, H., Fabritius, A.L., Salonen, T., Rapola, J., Gentile, M., Kopra, O., Peltonen, L., 2005. Mice with Ppt1 Dex4 mutation replicate the INCL phenotype and show an inflammation-associated loss of interneurons. Neurobiol. Dis. 18, 226 – 241. Jarvela, I., Lehtovirta, M., Tikkanen, R., Kyttala, A., Jalanko, A., 1999. Defective intracellular transport of Cln3 is the molecular basis of Batten disease (JNCL). Hum. Mol. Genet. 8, 1091 – 1098. Katz, M.L., Shibuya, H., Liu, P.C., Kaur, S., Gao, C.L., Johnson, G.S., 1999. A mouse gene knockout model for juvenile ceroid-lipofuscinosis (Batten disease). J. Neurosci. Res. 57, 551 – 556. Kim, Y., Ramirez-Montealegre, D., Pearce, D.A., 2003. A role in vacuolar arginine transport for yeast Btn1p and for human Cln3, the protein defective in Batten disease. Proc. Natl. Acad. Sci. U. S. A. 100, 15458 – 15462. Kopra, O., Vesa, J., Von Schantz, C., Manninen, T., Minye, H., Fabritius, A.L., Rapola, J., Van Diggelen, O.P., Saarela, J., Jalanko, A., Peltonen, 835 L., 2004. A mouse model for Finnish variant late infantile neuronal ceroid lipofuscinosis, CLN5, reveals neuropathology associated with early aging. Hum. Mol. Genet. 13, 2893 – 2906. Kriscenski-Perry, E., Applegate, C.D., Serour, A., Mhyre, T.R., Leonardo, C.C., Pearce, D.A., 2002. Altered flurothyl seizure induction latency, phenotype, and subsequent mortality in a mouse model of juvenile neuronal ceroid lipofuscinosis/Batten disease. Epilepsia 43, 1137 – 1140. Lam, H.H.D., Mitchison, H.M., Greene, N.D.E., Nussbaum, R.L., Mobley, W.C., Cooper, J.D., 1999. Pathologic involvement of interneurons in mouse models of neuronal ceroid lipofuscinosis. Abstr. -Soc. Neurosci. 25, 1593. Lauronen, L., Heikkila, E., Autti, T., Sainio, K., Huttunen, J., Aronen, H.J., Korvenoja, A., Ilmoniemi, R.J., Santavuori, P., 1997. Somatosensory evoked magnetic fields from primary sensorimotor cortex in juvenile neuronal ceroid lipofuscinosis. J. Child Neurol. 12, 355 – 360. Lauronen, L., Munroe, P.B., Jarvela, I., Autti, T., Mitchison, H.M., O’Rawe, A.M., Gardiner, R.M., Mole, S.E., Puranen, J., Hakkinen, A.M., Kirveskari, E., Santavuori, P., 1999. Delayed classic and protracted phenotypes of compound heterozygous juvenile neuronal ceroid lipofuscinosis. Neurology 52, 360 – 365. Mao, Q., Foster, B.J., Xia, H., Davidson, B.L., 2003. Membrane topology of Cln3, the protein underlying Batten disease. FEBS Lett. 541, 40 – 46. Mitchison, H.M., Bernard, D.J., Greene, N.D., Cooper, J.D., Junaid, M.A., Pullarkat, R.K., de Vos, N., Breuning, M.H., Owens, J.W., Mobley, W.C., Gardiner, R.M., Lake, B.D., Taschner, P.E., Nussbaum, R.L., 1999. Targeted disruption of the Cln3 gene provides a mouse model for Batten disease. The Batten mouse model consortium. Neurobiol. Dis. 6, 321 – 334. Mitchison, H.M., Lim, M.J., Cooper, J.D., 2004. Selectivity and types of cell death in the neuronal ceroid lipofuscinoses. Brain Pathol. 14, 86 – 96. Oliet, S.H., Piet, R., Poulain, D.A., 2001. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science 292, 923 – 926. Oswald, M.J., Kay, G.W., Palmer, D.N., 2001. Changes in GABAergic neuron distribution in situ and in neuron cultures in ovine (OCL6) Batten disease. Eur. J. Paediatr. Neurol. 5, 135 – 142 (Suppl.). Oswald, M.J., Palmer, D.N., Kay, G.W., Shemilt, S.J.A., Rezaie, P., Cooper, J.D., in press. Glial activation spreads from specific cerebral foci and precedes neurodegeneration in presymptomatic ovine neuronal ceroid lipofuscinosis (CLN6). Neurobiol. Dis. doi:10.1016/j.nbd.2005.01.025. Paxinos, G., Franklin, K.B.J., 2001. The Mouse Brain in Stereotaxic Coordinates, 2nd ed. Academic Press, San Diego. Pearce, D.A., Ferea, T., Nosel, S.A., Das, B., Sherman, F., 1999. Action of BTN1, the yeast orthologue of the gene mutated in Batten disease. Nat. Genet. 22, 55 – 58. Piet, R., Poulain, D.A., Oliet, S.H., 2002. Modulation of synaptic transmission by astrocytes in the rat supraoptic nucleus. J. Physiol. (Paris) 96, 231 – 236. Pontikis, C.C., Cella, V.C., Parihar, N., Lim, M.J., Chakrabarti, S., Mitchison, H.M., Mobley, W.C., Rezaie, P., Pearce, D.A., Cooper, J.D., 2004. Late onset neurodegeneration in the Cln3 / mouse model of juvenile neuronal ceroid lipofuscinosis is preceded by low level glial activation. Brain Res. 1023, 231 – 242. Raivich, G., Bohatschek, M., Kloss, C.U., Werner, A., Jones, L.L., Kreutzberg, G.W., 1999. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res. 30, 77 – 105. Seigel, G.M., Lotery, A., Kummer, A., Bernard, D.J., Greene, N.D., Turmaine, M., Derksen, T., Nussbaum, R.L., Davidson, B., Wagner, J., Mitchison, H.M., 2002. Retinal pathology and function in a Cln3 knockout mouse model of juvenile neuronal ceroid lipofuscinosis (Batten disease). Mol. Cell. Neurosci. 19, 515 – 527. Simard, M., Nedergaard, M., 2004. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience 129, 877 – 896. Sleat, D.E., Wiseman, J.A., El-Banna, M., Kim, K.H., Mao, Q., Price, 836 C.C. Pontikis et al. / Neurobiology of Disease 20 (2005) 823 – 836 S., Macauley, S.L., Sidman, R.L., Shen, M.M., Zhao, Q., Passini, M.A., Davidson, B.L., Stewart, G.R., Lobel, P., 2004. A mouse model of classical late-infantile neuronal ceroid lipofuscinosis based on targeted disruption of the CLN2 gene results in a loss of tripeptidyl-peptidase I activity and progressive neurodegeneration. J. Neurosci. 24, 9117 – 9126. Streit, W.J., 2000. Microglial response to brain injury: a brief synopsis. Toxicol. Pathol. 28, 28 – 30. Streit, W.J., 2002. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 40, 133 – 139. Tackmann, W., Kuhlendahl, D., 1979. Evoked potentials in neuronal ceroid lipofuscinosis. Eur. Neurol. 18, 234 – 242. Tyynelä, J., Suopanki, J., Santavuori, P., Baumann, M., Haltia, M., 1997. Variant late infantile neuronal ceroid-lipofuscinosis: pathology and biochemistry. J. Neuropathol. Exp. Neurol. 56, 369 – 375. Tyynelä, J., Cooper, J.D., Khan, M.N., Shemilt, S.J.A., Haltia, M., 2004. Hippocampal pathology in the human neuronal ceroid-lipofuscinoses: distinct patterns of storage deposition, neurodegeneration and glial activation. Brain Pathol. 14, 349 – 357. Vanhanen, S.L., Sainio, K., Lappi, M., Santavuori, P., 1997. EEG and evoked potentials in infantile neuronal ceroid-lipofuscinosis. Dev. Med. Child Neurol. 39, 456 – 463. Vanhanen, S.L., Puranen, J., Autti, T., Raininko, R., Liewendahl, K., Nikkinen, P., Santavuori, P., Suominen, P., Vuori, K., Hakkinen, A.M., 2004. Neuroradiological findings (MRS, MRI, SPECT) in infantile neuronal ceroid-lipofuscinosis (infantile CLN1) at different stages of the disease. Neuropediatrics 35, 27 – 35. Vercruyssen, A., Martin, J.J., Ceuterick, C., Jacobs, K., Swerts, L., 1982. Adult ceroid-lipofuscinosis: diagnostic value of biopsies and of neurophysiological investigations. J. Neurol., Neurosurg. Psychiatry 45, 1056 – 1059. West, M.J., Slomianka, L., Gundersen, H.J., 1991. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 231, 482 – 497.