* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Nuclear Chemistry - Mona Shores Blogs
Survey
Document related concepts
Fallout shelter wikipedia , lookup
Nuclear and radiation accidents and incidents wikipedia , lookup
Ionizing radiation wikipedia , lookup
Background radiation wikipedia , lookup
Nuclear fusion wikipedia , lookup
Nuclear fusion–fission hybrid wikipedia , lookup
Nuclear fission product wikipedia , lookup
Nuclear fission wikipedia , lookup
Radioactive decay wikipedia , lookup
Technetium-99m wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear drip line wikipedia , lookup
Transcript
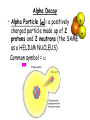
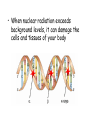
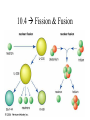
Nuclear Chemistry 10.1 Radioactivity • Radioactivity: process in which an atomic nucleus emits charged particles and energy • Radioisotope: any atom containing an unstable nucleus During nuclear decay, atoms of one element can change into atoms of a different element altogether Uranium – 238 decays into Thorium – 234 (also a radioisotope) • Nuclear Radiation: charged particles and energy that are emitted from the nuclei of radioisotopes • Common nuclear radiation types – alpha particle – beta particle – gamma rays Alpha Decay • Alpha Particle (): a positively charged particle made up of 2 protons and 2 neutrons (the SAME as a HELIUM NUCLEUS) Common symbol = Example of alpha decay of uranium – 238 Dangers of Nuclear Radiation • Least penetrating type of nuclear radiation, can be stopped by sheet of paper or clothing Beta Decay • Beta Particle (): an electron emitted by an unstable nucleus – Written as: • Assigned atomic # of -1 mass of 0 (zero) • How can a nucleus (which is positive), emit a negatively charged particle? During beta decay, a neutron decomposes into a proton and an e- • Proton stays trapped in the nucleus, e- released Example of beta decay of thorium –234 • Product isotope has 1 proton more and 1 neutron fewer than the reactant isotope • Mass number of the isotopes are equal because the emitted beta particle has essentially NO MASS • Beta particles pass through paper, but stopped by thin sheet of metal Gamma Decay • Gamma Ray (): a penetrating ray of energy emitted by an unstable nucleus Gamma Decay • NO mass and NO charge • During Gamma Decay: • Atomic number and mass number of the atom remains the same • Energy of nucleus decreases • Gamma decay often accompanied by alpha or beta decay • Example of thorium – 234 emitting both beta particles and gamma rays as it decays: • Gamma rays much more penetrating – takes several centimeters of lead or several meters of concrete to stop gamma radiation Effects of Nuclear Radiation • Background Radiation: nuclear radiation that occurs naturally in the environment • When nuclear radiation exceeds background levels, it can damage the cells and tissues of your body Effects of Nuclear Radiation • Nuclear radiation can ionize atoms (when cells are exposed to nuclear radiation, the bonds holding together proteins and DNA molecules may break cells may no longer function properly) Effects of Nuclear Radiation • , , and are all forms of ionizing radiation • The extent of the damage of external nuclear radiation is dependent on the penetrating power of the radiation… • Beta particles cause more damage than alpha particles, but less than gamma rays • Gamma rays can penetrate deeply into the human body, potentially exposing all organs to ionization damage Detecting Nuclear Radiation • Although you can’t see, hear, or feel the radioactivity around you, scientific instruments can measure nuclear radiation • Geiger Counters • Film Badges 10.2 Rates of Nuclear Decay Nuclear Decay • By studying the radioactive nuclei of an object we can determine how old the object is. • Because most materials contain at least trace amounts of radioisotopes, scientists can estimate how old they are based on rates of nuclear decay. Half-Life • Half-life: the time required for one half of a sample of a radioisotope to decay – After one half-life, half of the atoms in a radioactive sample have decayed, while the other half remain unchanged – After two half-lives, half of the remaining have decays, leaving one quarter of the original sample unchanged Half-Life Example • Iodine Half-life= 8.07 days – After one half-life (8.07 days) half of the original sample remains – After 2 half-lives (16.14 days) one quarter of the original remains – After 3 half-lives (24.21 days) one half of one quarter remains, or 1/8 (one eighth) – …and so on Half-Lives Vary • Half-lives can vary from fractions of a second to billions of years • Unlike chemical reaction rates, which vary with the conditions of a reaction, nuclear decay rates are constant!!! Radioactive Dating • Method used for determining the age of objects using the half-lives of Carbon – 14 • Radiocarbon dating: determining the age of an object by comparing its carbon-14 levels with carbon-14 levels in the atmosphere. Radioactive Dating • Carbon-14 has a half-life of 5,730 years. • Carbon-14 is formed in the upper atmosphere when neutrons produced by cosmic rays collide with nitrogen-14 atoms. • The radioactive carbon-14 undergoes beta decay to form nitrogen-14. Using Carbon-14 to Date • Living organisms absorb the carbon (CO2) from the atmosphere, but when they die they stop absorbing it and the levels do not change. • From this point levels start to decrease as the radioactive carbon decays. • The levels in the object are then compared with levels in the atmosphere. Example: if an object has half the amount of carbon-14 in it as in the atmosphere, then we know the object is about 5, 730 years old (which is one half-life for carbon-14) Carbon-14 Dating • Carbon-14 or radiocarbon dating can be used to date any carbon-containing object less than 50,000 years old. – After this point, there is too little carbon-14 left to be measurable • Objects older than this use radioisotopes with longer half-lives • The older the object the lower the levels of radioisotopes present 10.3 Artificial Transmutation Transmutation • Transmutation: the conversion of atoms of one element to atoms of another. • It involves a nuclear change, not a chemical change. • Transmutations can either occur naturally (nuclear decay) or artificially. • Scientists can perform artificial transmutations by bombarding atomic nuclei with high-energy particles such as protons, neutrons, or alpha particles. Transuranium Elements • Transuranium Elements: Elements with atomic numbers greater than 92 (uranium) – All transuranium elements are radioactive and generally not found in nature • Scientists can create a transuranium element by the artificial transmutation of a lighter element • Useful transuranium elements – Americium-241: used in smoke detectors – Plutonium-238: energy source for space probes Particle Accelerators • Sometimes transmutations will not occur unless the bombarding particles are moving at extremely high speeds. • To achieve these high speeds scientists use particle accelerators. Particle Accelerator • These accelerators move charged particles at speeds very close to the speed of light • The particles are then guided toward a target, where they collide with atomic nuclei and transmutations are allowed to occur • These collisions have also lead to the discovery of more subatomic particles – Quarks: protons and neutrons are made up of these even smaller particles Large Hadron Collider (LHC) 10.4 Fission & Fusion Question • What holds the nucleus together? • It’s full of positive particles, so why don’t they push each other away? • What keeps the protons and neutrons together? • Clearly, there must be an attractive force that binds the particles Answer • Strong Nuclear Force: the attractive force that binds protons and neutrons together in the nucleus – Over very short distances, the strong nuclear force is much greater than the electric forces among protons Forces in the Atom Electric Force • Question: What determines the strength of the electric force? • Answer: The number of protons Electric Force • The greater the number of protons, the greater is the electric force that repels the protons • Larger nuclei have a stronger repulsive force than a smaller nuclei • As a result, the nucleus will become unstable (or radioactive) when the strong nuclear forces can’t overcome the repulsive electric forces among protons. Nucleus Size & Radioactivity • Because of the size issue, there is a point beyond which all elements are radioactive. • Once they become large enough, the repulsive forces overcome. This occurs with all nuclei with 83 or more protons. • Therefore, all elements with an atomic number greater than 83 are radioactive! Fission • FISSION: the splitting of an atomic nucleus into two smaller parts • In nuclear fission, tremendous amounts of energy can be produced from very small amounts of mass Converting Mass into Energy • During a fission reaction, some of the mass of the reactants is lost! • The Law of Conservation of Mass says this is illegal, highly illegal! • This “lost” mass is converted into energy! Converting Mass into Energy • Since we bent the law a little…we use a revised version of the law: Law of Conservation of Mass and Energy – It basically says: The total amount of mass and energy remains constant!!! E= 2 mc • 30 years before the discovery of fission, Albert Einstein introduced the mass-energy equation. • E=mc2describes the relationship between mass and energy: – E = energy – m = mass – c = the speed of light (3.0 x 108m/s) • It shows that the conversion of a small amount of mass releases an ENORMOUS amount of energy. Lots of Energy!!! • Example: the explosion of the first atomic bomb contained 5kg of plutonium, but created an explosion equivalent to18, 600 tons of TNT!!! • So, since we bent the law a little, just a little, we use a revised version of the law: – This law is referred to as the Law of Conservation of mass and energy. • It basically says: – THE TOTAL AMOUNT OF MASS AND ENERGY REMAINS CONSTANT! Chain Reaction • Nuclear fission reactions act like rumors being spread throughout school: • One person tells a few friends, they tell a few friends, and on and on… • During a fission reaction each reactant nucleus splits into 2 smaller nuclei and releases 2-3 neutrons. • If one of these neutrons is absorbed by another nucleus, fission can result again, releasing more neutrons. Triggering a Chain Reaction • CHAIN REACTION: neutrons released during the splitting of an initial nucleus trigger a series of nuclear fissions. • Uncontrolled chain reactions occur when each released neutron is free to cause other fissions Chain Reaction Chain Reaction • Nuclear weapons are designed to produce uncontrolled chain reactions • In order for a chain reaction to keep going, the nucleus that splits needs to produce one neutron that causes the fission of another nucleus – The material reacting uncontrolled needs to have a critical mass. – CRITICAL MASS: the smallest possible mass of a fissionable material that can sustain a chain reaction. Fusion • Another type of nuclear reaction can release huge amounts of energy is fusion: • FUSION: a process in which the nuclei of two atoms combine to form a larger nucleus. • Just like fission, a small fraction of the mass is converted into energy Example of Fusion • The sun and stars are powered by the fusion of hydrogen into helium – Fusion requires extremely high temperatures where matter exists as plasma. • This is a problem for scientists wanting to use fusion for an energy source – They cannot get high enough temperatures and have trouble containing plasma here on Earth Fusion in the Core