* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download radioactive decay - Aurora City Schools
Survey
Document related concepts
Background radiation wikipedia , lookup
Nuclear fusion wikipedia , lookup
Ionizing radiation wikipedia , lookup
Nuclear fission product wikipedia , lookup
Nuclear fission wikipedia , lookup
Technetium-99m wikipedia , lookup
Isotopic labeling wikipedia , lookup
Radioactive decay wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Nuclear drip line wikipedia , lookup
Transcript
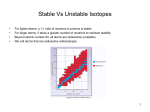
Ch 9 Nuclear Decay • Review from ch 4… • Atomic number (Z) tells you number of protons – Always the same for an element; change the atomic number and you change the element • Mass number (A) tells you number of protons + neutrons – can change as number of neutrons changes for each isotope Isotopes • An element with a different number of neutrons • Because has same number of protons, still that element and has all chem/phys properties • Write isotopes using atomic # & mass # Radioactivity • Elements become unstable over time…it’s a natural process • To become more stable, they emit energy or matter or both • These matter/energy emissions are called nuclear radiation • The process is called nuclear decay or radioactive decay Alpha (a) Decay • When 2 protons and two neutrons are given off • Basically the nucleus of a Helium atom • Decreases the atomic number by 2 and mass number by 4 4 2 He Beta (b) Decay • When an electron is given off from the nucleus • A neutron decays into a proton (which stays) and an electron which leaves the atom • Doesn’t change the mass number, atomic number goes up by 1 0 -1 e Gamma (g) Radiation • No mass, so atomic number and mass number don’t change • Just a photon of light energy in the gamma wavelengths g or 0 0 g Mass/energy • Alpha particles – Have the most mass and the least energy – Barely pass through paper • Beta particles – Less mass and more energy – Stopped by 3mm metal foil, 10cm wood • Gamma radiation – has no mass and the most energy – Stopped by 60cm foil or 7 cm lead – Are most damaging • All three ionize atoms (steal electrons) as they move through materials. This is how the damage is done Why decay? • 2 forces inside the nucleus – Repulsion force: protons in the nucleus trying to stay away from each other (one kind of Coulomb force) • Acts over far distances so 1 proton on one side of the nucleus pushes ones on the other side away – Strong Nuclear Force: one of 4 fundamental forces holds nucleus together • Acts over short distances…only on particle next to it • That’s why many more neutrons at higher atomic number…more strong nuclear force Why decay? • If those two forces are not balanced, the nucleus will emit particles until it becomes more stable Decay Equations • Decay equations are just like chemical equations – Reactant on left – Products on right – All particles have to balance Half Life • Nuclei decay at a steady, measurable rate called the half life (t1/2 ) • Defined as the time it takes for only ½ the original to remain (and ½ to decay) • It decays into another isotope of the same element, or into another element. It doesn’t just disappear. Half life • You use the ratio of the original product to the decayed product to get a % that has decayed. • Use that percent and the half life to tell how old something is