* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chromatin Impacts on Human Genetics
Gene desert wikipedia , lookup
Genetic engineering wikipedia , lookup
Point mutation wikipedia , lookup
Minimal genome wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
Oncogenomics wikipedia , lookup
Genome evolution wikipedia , lookup
Gene nomenclature wikipedia , lookup
Ridge (biology) wikipedia , lookup
Public health genomics wikipedia , lookup
Gene therapy wikipedia , lookup
Cancer epigenetics wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Epigenetics wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
X-inactivation wikipedia , lookup
Gene expression programming wikipedia , lookup
History of genetic engineering wikipedia , lookup
Epigenomics wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Dominance (genetics) wikipedia , lookup
Genome (book) wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Gene expression profiling wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Designer baby wikipedia , lookup
Microevolution wikipedia , lookup
Epigenetics of human development wikipedia , lookup
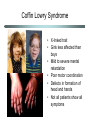
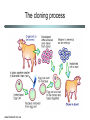
Chromatin Impacts in Human Genetics Chromatin-mediated influences • Gametic (parental) imprinting • Regulation of gene expression • Developmental programming Gametic (parental) imprinting • Gametic imprinting is the differential expression of gene that depends on which parent donated the gene • Most genes are expressed from both maternally and paternally inherited chromosomes. • However, some genes are expressed only from the maternally inherited allele, whereas others are expressed only from the paternally inherited allele. Successful development requires both maternal and paternal genes Diseases tracked to imprinting Prader Willi Syndrome • Small hands and feet • Underactive gonads, tiny external genitals • Short stature • Mentally retarded • Slow-moving • Compulsive overaters • Obese Diseases tracked to imprinting Angelman Syndrome • “Happy puppet” • Severe mental retardation • Absence of speech • Happy disposition • Excessive laughing • Hyperactive, with jerky repetitive motions • Red cheeks, large jaw and mouth Patterns of inheritance Both diseases are associated with deletions in the same region of chromosome 15 Parental Origin Mother Father Phenotype Normal allele Normal allele Normal Normal allele Deletion Prader-Willi Deletion Normal Angelman Genes in imprinted region of Chr 15 Blue: Prader Willi gene candidates Failure to inherit a good allele from dad results in disease. Paternal allele is expressed. Maternal allele is silent. Pink: Angelman gene candidates Failure to inherit a good allele from mom results in disease Maternal allele is expressed. Paternal allele is silent. Gametic imprinting is epigenetic • For imprinted genes, one allele is expressed and the other is silent. • The silent alleles typically show high levels of DNA methylation and tightly packed chromatin, consistent with their transcriptional inactivity. • The expressed alleles are unmethylated and associated with loosely packed chromatin. What we don’t know about imprinting • What targets a gene for imprinting? – Why are some genes expressed from both alleles and other expressed from only one allele? • How are the imprints imposed? – Do males and females have different mechanisms for imprinting genes? Rett Syndrome • • • • Nature Genetics (1999) 23:127-128 X-linked trait Mainly girls affected Normal at birth At 6-18 months, begin losing purposeful movement • Persistent wringing of hands • Loss of speech, gait • Mental retardation ensues Rett’s is due to defect in MeCP2 • Methyl-cytosine binding protein 2 (MeCP2) binds methylated DNA and recruits binding of a histone deacetylase • Normal role is tightening chromatin packing, leading to gene silencing Why is the phenotype neurological? • The phenotype suggests that the targets are genes in the brain • Normal neurological differentiation requires silencing of MeCP2 gene target(s) • The target(s) of MeCP2 are not known • Mice have a gene that is homologous to MeCP2 • Knocking out the gene in mouse gives a phenotype similar to human Rett’s • This model offers good experimental system for studying the human disease Nature Genetics (2001) 27:332-336 Mouse model for Rett’s Male mice with MeCP2 knockout develop normally for a while (middle), but at 6 weeks of age, they begin to develop neurological symptoms, such as hindlimb clasping (right). Coffin Lowry Syndrome • X-linked trait • Girls less affected than boys • Mild to severe mental retardation • Poor motor coordination • Defects in formation of head and hands • Not all patients show all symptoms Coffin Lowry defect in Rsk2 gene • Rsk2 codes for a protein kinase, which phosphorylates proteins that participate in stimulating cell division and cellular differentiation. • The Rsk2 protein associates with a histone acetyltransferase. Together, these proteins phosphylate and acetylate histone H3. • Modification of histone H3 is associated with activation of a suite of genes, whose identity is not yet known. • When Rsk2 is not functional, expression of the target genes is repressed, thus leading to disease. Chromatin and Cloning • During differentiation of cells, many changes in chromatin occur. • Cloning from adult cells requires re-programming to return the cell to an embryonic state. • Re-programming requires erasing the differentiationspecific chromatin changes. Cloning anomalies • Initial successes in cloning are being reevaluated. • Observations include: – Dolly, the first cloned sheep, at age 5 years is fat. – Cloned mice also tend to be overweight. – Some cow clones have been born with abnormally large hearts and lungs. The cloning process www.bioteach.ubc.ca What’s the problem with cloning? • After nuclear transfer, the donor nucleus often fails to re-establish an embryonic pattern of gene expression. • New studies from R. Jaenisch’s lab show that this is due to the differentiation and DNA methylation state of the donor nuclei. – Neural stems cells make better donors than differentiated neural cells. – Cells from DNA methylation mutants make better donors than normal cells. • Conclusion: Successful cloning requires reprogramming of chromatin modifications. Summary • Chromatin modifications play key roles in many important processes. – Parent-of-origin (imprinting) differences in gene expression – Activation and repression of gene expression during development – Differentiation of cells during development • Disruption of normal chromatin modification can result in disease.