* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Protein and Lipid Catabolism
Biosynthesis wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Butyric acid wikipedia , lookup
Metalloprotein wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Mitochondrion wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Photosynthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Phosphorylation wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biochemistry wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Electron transport chain wikipedia , lookup
Microbial metabolism wikipedia , lookup
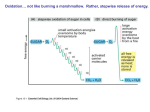
Tricarboxcylic acid cycle • Anaerobic, cell membrane or mitochondria • Each pyruvate gives up its carbon as CO2 – 6 total • Oxaloacetate is regenerated with every turn – Pick up molecule • 2 ATP are produced – Substrate level phosphorylation TCA cycle occurs twice per glucose Net yield of product per glucose molecule: •6 CO2 •2 ATP •8 NADH = 3 ATP •2 FADH2 = 2 ATP ELECTRON TRANPORT • Aerobic or anaerobic • Final electron acceptor: – aerobic respiration - oxygen – anaerobic respiration - CO2, NO3-, SO42 • Inner mitochondrial membrane or plasma membrane • Electrons move down chain and set up H+ gradient – drives chemiosmosis Electron transport systems consist of separate protein complexes Oxidative Phosphorylation – series of redox reactions creating a stepwise release of energy Proton Motive Force generated by chemical and electrical gradient Proton flow across membrane is exerogonic Using the PMF, ATP synthesis is catalyzed by ATP synthase (ATPase), through a process called chemiosmosis Complete Aerobic Catabolism of Glucose • C6H12O6 + 6O2 + 36ADP + 36P → 6CO2 + 6H2O + 36ATP – (eukaryote) • C6H12O6 + 6O2 + 38ADP + 38P → 6CO2 + 6H2O + 38ATP – (prokaryote) Typical net energy yield: 36 ATP for eukaryotes 38 ATP for prokaryotes By-products of aerobic respiration are H2O and CO2 Substrate-Level Phosphorylation – 2 ATP (net gain Glycolysis) – 2 ATP (TCA cycle) – 4 Total from substrate-level phosphorylation Oxidative Phosphorylation – 6 ATP (NADH Glycolysis) – 28 ATP (NADH/FADH2 TCA cycle) – 34 total from oxidative phosphorylation Total ATP gain ~ 36 to 38 Anaerobic Respiration Many compounds can serve as terminal electron acceptors • E.coli – Nitrate reduction – N03- + 2e- + 2H+ N02-+ H20 • Paracoccus, Bacillus and Pseudomonas – Denitrification – N03N02- NO N2O N2 Nitrate reduction and ammonification Denitrification in Paracoccus • Desulfovibrio – Reduce sulfate – acetate + SO4-2 + 3H+ 2CO2 + H2S + 2H2O • Archaea – Methanogens that reduce carbonate – HC03- + 4H2 + H+ CH4 + 3H2O • Common – Not associated with any one phylogenetic group – Except methanogenesis • Involves: – membrane system – generation of ion gradient – formation of ATP via ATP synthase • Less efficient than aerobic respiration – Electron acceptors have less positive reduction potentials than oxygen – lower energy yield Fermentation • Used by organisms that can’t respire – lack of suitable inorganic electron acceptor or lack of electron transport chain • Anaerobic; Occurs in the cytoplasm – Partial oxidation of substrate • NADH oxidized back to NAD+ • Uses organic compound as terminal electron acceptor – Typically pyruvate or derivative • NO oxidative phosphorylation so ATP yield is low • Lactic acid fermentation – pyruvate reduced to lactate – pyruvate accepts electrons and protons from NADH • Alcohol fermentation – pyruvate decarboxylated to form acetaldehyde – NADH transfers electrons and protons to acetaldehyde reducing it to ethanol Catabolism of Other Organic Compounds • Carbohydrates are the main energy source – glucose • Microbes may also utilize lipids and proteins – Both must be broken down into their individual components – Each component is oxidized separately Lipid Catabolism • Lipases • Fatty acids and glycerol – Fatty acid converted into acetyl CoA, enters TCA cycle – Glycerol converted into DHAP, enters glycolysis Protein Catabolism • Proteases • Amino acids – – – – can NOT be catabolized directly transamination decarboxylation dehydrogenation