* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download biomolecule
Survey
Document related concepts
Western blot wikipedia , lookup
Bottromycin wikipedia , lookup
Genetic code wikipedia , lookup
Self-assembling peptide wikipedia , lookup
Expanded genetic code wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Biosynthesis wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Endomembrane system wikipedia , lookup
Circular dichroism wikipedia , lookup
Protein structure prediction wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein adsorption wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
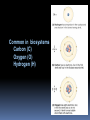
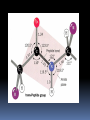
Biochemistry An Introduction to the Chemistry of Life for Biology Students Composition of living matter “Living things are composed of lifeless molecules” (Albert Lehninger) “Chemistry is the logic of biological phenomena” (Garrett and Grisham) Organisms are complicated and highly organized Biological structures serve functional purposes Living systems are actively engaged in energy transformations Living systems have a remarkable capacity for self-replication Biomolecules: The Molecules of Life H, O, C and N make up 99% of atoms in the human body ELEMENT PERCENTAGE Oxygen 63 Hydrogen 25.2 Carbon 9.5 Nitrogen 1.4 Common in biosystems Carbon (C) Oxygen (O) Hydrogen (H) What property unites H, O, C and N and renders these atoms so appropriate to the chemistry of life? Answer: Their ability to form covalent bonds by electron-pair sharing. Types of chemical bonds Covalent bonds • Common in biosystems • Share a pair of electrons Ionic Bonds • Transfer an electron • Opposite charges attract Types of Chemical Bonds Hydrogen bonds • Weak partial bonds • Water surface tension Van der Waals forces Weak Functional groups Simple Molecules are the Units for Building Complex Structures Metabolites and Macromolecules Organelles Membranes The Unit of Life is the Cell Primary Organic Compounds (macromolecules) You are expected to learn the structure and functions of these organic compounds: 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Polymers ands Monomers Each of these types of molecules are polymers that are assembled from single units called monomers. Each type of macromolecule is an assemblage of a different type of monomer. Monomers Macromolecule Carbohydrates Monomer ( biomolecule) Monosaccharide Lipids Proteins Not always polymers; Hydrocarbon chains Amino acids Nucleic acids Nucleotides How do monomers form polymers? In condensation reactions (also called dehydration synthesis), a molecule of water is removed from two monomers as they are connected together. Hydrolysis In a reaction opposite to condensation, a water molecule can be added (along with the use of an enzyme) to split a polymer in two. Properties of Biomolecules Macromolecules and Their Building Blocks Have a “Sense” or Directionality Macromolecules are Informational Biomolecules Have Characteristic ThreeDimensional Architecture Weak Forces Maintain Biological Structure and Determine Biomolecular Interactions Cell hierarchy Biomolecules combine to form macromolecules. And macromolecules combine non covalently to form supramolecules, such as: Supramolecule Biomolecules Biomolecule Lipo proteins lipids proteins Ribosomes Nucleic acids proteins Glycolipids Sugar lipids Cell hierarchy Finally, at the higher level of organization of the cell structure, supramolecules are further assembled into call organelles. ( Nuclei, mitochondria, chloroplasts, etc..) Macromolecules: a) Carbohydrates Carbohydrates are made of carbon, hydrogen, and oxygen atoms, always in a ratio of 1:2:1. Carbohydrates are the key source of energy used by living things. The building blocks of carbohydrates are sugars, such as glucose and fructose. Carbohydrates What do the roots mono-, di-, oligo-, and poly mean? Each of these roots can be added to the word saccharide to describe the type of carbohydrate you have. How do two monosaccharides combine to make a polysaccharide? Polysaccharides Lipids Lipids are molecules that consist of long hydrocarbon chains. Attaching the three chains together is usually a glycerol molecule. Lipids are NONpolar. Saturated vs. Unsaturated Fat Proteins Proteins are building blocks of structures called amino acids. Proteins are what your DNA codes to make . A peptide bond forms between amino acids by dehydration synthesis. Protein Structure Level Primary Secondary Tertiary Quaternary Description The amino acid sequence Helices and Sheets Disulfide bridges Multiple polypeptides connect