* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cellular Respiration: Harvesting Chemical Energy
Survey
Document related concepts
Evolution of metal ions in biological systems wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Mitochondrion wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Light-dependent reactions wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Electron transport chain wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Transcript
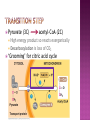
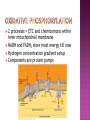
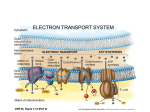
Chapter 9 All energy ultimately comes from the sun Energy flows into an ecosystem as sunlight and leaves as heat Chemical components are recycled Energy is released from the transfer of e-’s Oxidation is a loss of (hydrogen) e-’s Reduction is a gain of (hydrogen) e-’s Reduce (+) charge of an atom by adding a (-) charge eLEO goes GER or OIL RIG Always occur together Organic molecules have high abundance of hydrogen Energy released as O2 oxidizes (accepts e-’s from) H2 e-’s travel with a proton (hydrogen atom) PE lost as e-’s ‘fall’ down an energy gradient (move towards more EN molecule) Lower energy state from less complex molecules Enzymes needed to facilitate in animals because of energy of activation (EA) Glucose (C6H12O6) is oxidized during cellular respiration Occurs in steps to effectively access and use energy (avoids explosions) e-’s transferred to an electron carrier, not directly to O2 Coenzyme NAD+ as an oxidizing agent (e- acceptor) Dehydrogenase removes a pair of hydrogen atoms (2 e-’s and 2 protons) With no intermediates reaction = explosion Little PE lost with e- transfer to NAD+ Each NADH stores energy for ‘fall’ to O2 (final e- acceptor) Electron transport chain, proteins within inner mitochondrial membrane, controls Each carrier more EN than previous 3 metabolic stages Glycolysis: in cytosol, breaks down glucose into pyruvate Citric acid cycle: in mitochondrial matrix, oxidizes pyruvate to create CO2 Oxidative phosphorylation: mitochondrial matrix, e-’s to O2 and H+ = H2O and synthesizes ATP Makes 36-38 ATP Glucoses stores 686 kcal/mol of energy in bonds ATP has 7.3 kcal/mol Single, large unit of energy broken into smaller, more usable forms Glucose (6C) 2 pyruvate (3C) No CO2 released Substrate-level phosphorylation Can occur with or without O2 O2 needed for citric acid cycle No O2 then fermentation Pyruvate (3C) acetyl-CoA (2C) High energy product so reacts exergonically Decarboxylation is loss of CO2 ‘Grooming’ for citric acid cycle (2 C’s) Within mitochondria Acetyl-CoA ATP + 3 NADH + FADH2 + CO2 Intermediate 2 steps Acetyl-CoA becomes citrate CO2 and NADH leave as number of carbons reduces to 4 FADH2 and an additional NADH leave Oxaloacetate joins 2nd acetyl-CoA to reform citrate Cycle repeats turns per 1 glucose Oxaloacetate (4C’s) Citrate (6 C’s) (4 C) molecule 2 processes = ETC and chemiosmosis within inner mitochondrial membrane NADH and FADH2 store most energy till now Hydrogen concentration gradient setup Components are protein pumps NADH and FADH2 bring e-’s to ETC complex Oxidized when they lose e- to a lower neighbor (higher EN) No direct ATP, control of energy release from fuel to oxygen (final acceptor) Smaller, more manageable energy sources FADH2 adds to lower energy level Produce third less energy for ATP synthesis Sets up a H+ concentration gradient Energy stored as H+ gradient across a membrane drives cellular work H+ ions pumped out by ETC Sets up the gradient to drives ATP synthesis ATP synthase is a protein complex that makes ATP from ADP and Pi Endergonic reaction of ATP synthesis is coupled with exergonic ETC C6H12O6 + 6O2 1 NADH = 3 ATP 1 FADH2 = 2 ATP 6H2O + 6CO2 + 36-38 ATP + heat Anaerobic respiration ETC, but O2 not final e- acceptor Fermentation Primary purpose is to recycle NADH to NAD+ not ATP production Glycolysis Pyruvate picks up e-’s (reduced) Occurs because NAD+ is oxidizer, not O2 NAD+ recycling NADH transfers e-’s to pyruvate to recycle NAD+ Alcohol Pyruvate to ethanol Lose CO2 then reduced by NADH In bacteria and yeast; makes bread, beer, and wine Lactic fermentation acid fermentation Pyruvate directly by NADH to lactate No CO2 released To make cheese and yogurt