* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Enzyme Activity
Photoredox catalysis wikipedia , lookup
Chemical biology wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Process chemistry wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Thermal spraying wikipedia , lookup
Rate equation wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Stoichiometry wikipedia , lookup
Asymmetric induction wikipedia , lookup
Restriction enzyme wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Biochemistry wikipedia , lookup
Scanning electrochemical microscopy wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Click chemistry wikipedia , lookup
Self-assembled monolayer wikipedia , lookup
Metalloprotein wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Biosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Catalytic triad wikipedia , lookup
Reaction progress kinetic analysis wikipedia , lookup
Transition state theory wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
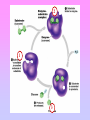
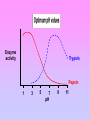
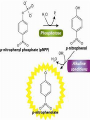
Enzymes: Nothing works without enzymes! • How important are enzymes? – all chemical reactions in living organisms require enzymes to work enzyme • building molecules – synthesis enzymes • breaking down molecules We can’t live without enzymes! – digestive enzymes – enzymes speed up reactions • “catalysts” + enzyme + Enzymes aren’t used up • Enzymes are not changed by the reaction – used only temporarily – re-used again for the same reaction with other molecules substrate active site product enzyme • Most enzymes are Proteins (tertiary and quaternary structures) • Act as Catalyst to accelerates a reaction • Are specific for what they will catalyze It’s shape that matters! • Lock & Key model – shape of protein allows enzyme & substrate to fit – specific enzyme for each specific reaction 2 1 3 The Lock and Key Hypothèses Fit between the substrate and the active site of the enzyme is exact Like a key fits into a lock Temporary structure called the enzyme-substrate complex formed Products have a different shape from the substrate Once formed, they are released from the active site Leaving it free to become attached to another substrate The Lock and Key Hypothesis S E E E Enzymesubstrate complex P P © 2007 Paul Billiet ODWS Reaction coordinate Enzyme may be used again Enzyme Kinetics Equation The active site • One part of an enzyme, the active site, is particularly important • The shape and the chemical environment inside the active site to proceed more easily © H.PELLETIER, M.R.SAWAYA ProNuC Database Chemical reactions Chemical reactions need an initial input of energy = THE ACTIVATION ENERGY During this part of the reaction the molecules are said to be in a transition state (ES) Making réactions go faster Increasing the temperature make molecules move faster Biological systems are very sensitive to temperature changes. Enzymes can increase the rate of reactions without increasing the temperature. They do this by lowering the activation energy. Factors affecting Enzymes substrate concentration pH temperature inhibitors 1- Substrate concentration: Reaction velocity Substrate concentration • The increase in velocity is proportional to the substrate concentration © 2007 Paul Billiet ODWS Substrate concentration: Vmax Reaction velocity Substrate concentration • Faster reaction but it reaches a saturation point when all the enzyme molecules are occupied. Vmax © 2007 Paul Billiet ODWS pH • Extreme pH levels will produce denaturation – most human enzymes = pH 6-8 • depends on where in body • pepsin (stomach) = pH 3 • trypsin (small intestines) = pH 8 • The structure of the enzyme is changed • The active site is distorted and the substrate molecules will no longer fit in it Enzyme activity Trypsin Pepsin 1 3 5 7 pH 9 11 Temperature • Effect on rates of enzyme activity – Optimum temperature • human enzymes – 35°- 40°C (body temp = 37°C) – Raise temperature (boiling) • denature protein = unfold = lose shape – Lower temperature T° • molecules move slower • fewer collisions between enzyme & substrate Many are a lot lower, cold water fish will die at 30°C because their enzymes denature A few bacteria have enzymes very high temperatures up to 100°C Most enzymes however are fully denatured at 70°C Temperature reaction rate human enzymes 37° temperature What’s happening here?! Inhibitors Inhibitors are chemicals that reduce the rate of enzymic reactions. The are usually specific and they work at low concentrations. They block the enzyme but they do not usually destroy it. The effect of enzyme inhibition Irreversible inhibitors: Combine with the functional groups of the amino acids in the active site, irreversibly. Examples: nerve gases and pesticides, containing combine with serine residues in the enzyme. Reversible inhibitors: There are two categories 1. • • • Competitive: These compete with the substrate molecules for the active site. The inhibitor’s action is proportional to its concentration. Come over these problem by adding more substrate • Km : The addition of a competitive inhibitor increases the • • • • • observed Km for a given substrate. Therefore, in the presence of a competitive inhibitor, more substrate is needed to achieve Vmax: Competitive inhibitors do not alter Vmax. The effect of a competitive inhibitor is reversed by increasing [S]. high substrate concentration, the reaction velocity reaches the same Vmax as that observed in the absence of the inhibitor. This is because at the higher concentration the active site will be saturated with substrate which means the inhibitor cannot bind 2. Non-competitive: (allosteric effect) • These are not treated by the concentration of the substrate. • It inhibits by binding irreversibly to the enzyme but not at the active site. Examples • Cyanide combines with the Iron in the enzymes cytochrome oxidase. • Km : Non-competitive inhibitors do not interfere with the binding of substrate to enzyme. • Thus, the enzyme shows the same Km in the presence or absence of the non-competitive inhibitor. • Vmax: Increasing the concentration of substrate does not overcome non-competitive inhibition. • Non-competitive inhibitors therefore decrease the Vmax of the reaction. • Non-competitive inhibitors therefore simply reduce the amount of active enzyme so they decrease Vmax, but have no effect on Km Kompetitive Inhibition: Km Increases; no change in Vmax. Non-kompetitive inhibition: No Km change, but Vmax decreases Applications of inhibitors • Negative feedback: end point or end product inhibition • Poisons snake bite, nerve gases. • Medicine antibiotics © 2007 Paul Billiet ODWS Michaelis-Menten Equation Glossary • Active site: The region of an enzyme molecule which binds the substrate and carries out the catalytic reaction • Enzyme : A biological catalyst. Usually a globular protein molecule produced by living organisms that can speed up a specific chemical reaction without itself being destroyed or changed in any way. • K m: (Michaelis constant) The substrate concentration at which an enzyme catalysed reaction proceeds at half the maximum velocity. • V max: (Maximum velocity) The maximum initial velocity of an enzyme catalysed reaction; determined by increasing the substrate [S] until a constant rate of product formation is achieved (i.e. saturating substrate levels). • A CATALYST is anything that speeds up a chemical reaction that is occurring slowly ALP • Alkaline phosphatase • The enzymes phosphatase catalyze hydrolysis of phosphate esters to free inorganic phosphate • The measurement of ALP activity in vitro is based on artificial substrate used • The artificial substrate p-nitrophenylphosphate • the enzyme will hydrolysis it to P-nitrophenol +phosphate ion • P-nitrophenol is bright yellow and other reactant and product are colorless /; thus formation of product can measurement by specphotometer 400nm • The intensity of color indicator how much enzyme acted on substrate ALP is present in most human tissues • • • • • Bone Liver Spleen Kidney Intestine • Diagnostic of ALP significance : 1. Hepatic disorder 2. Rickets 3. Paget disease For enzymes… What matters? SHAPE!