* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Mitogen-activated protein kinase wikipedia , lookup
Metabolomics wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biochemical cascade wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Transcript
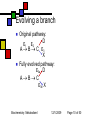
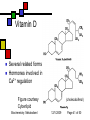
General Metabolism Principles; Nutrition Andy Howard Introductory Biochemistry 1 December 2009 Biochemistry: Metabolism I 12/1/2009 Metabolism depends strongly on cofactors We’ll attend to the reality that a lot of the versatility of enzymes depends on their incorporation of cofactors; and most vitamins are precursors of cofactors Biochemistry: Metabolism I 12/1/2009 Page 2 of 50 What we’ll discuss Post-translational modification Phosphorylation Other reversible PTMs How pathways evolve Oxidation-Reduction Reactions: Quantitation How we study metabolism (revisited) Biochemistry: Metabolism I Nutrition Macronutrients Micronutrients Specific cofactors and vitamins reconsidered Fat soluble vitamins Ascorbate 12/1/2009 Page 3 of 50 Phosphorylation’s effects Phosphorylation of an enzyme can either activate it or deactivate it Usually catabolic enzymes are activated by phosphorylation and anabolic enzymes are inactivated Example: glycogen phosphorylase is activated by phosphorylation; it’s a catabolic enzyme Biochemistry: Metabolism I 12/1/2009 Page 4 of 50 Amplification Activation of a single molecule of a protein kinase can enable the activation (or inactivation) of many molecules per sec of target proteins Thus a single activation event at the kinase level can trigger many events at the target level Biochemistry: Metabolism I 12/1/2009 Page 5 of 50 Other PTMs Are there other reversible posttranslational modifications that regulate enzyme activity? Yes: Adenylation of Y ADP-ribosylation of R Uridylylation of Y Oxidation of cysteine pairs to cystine Cis-trans isomerization of prolines Biochemistry: Metabolism I 12/1/2009 Page 6 of 50 Metabolism and evolution Metabolic pathways have evolved over hundreds of millions of years to work efficiently and with appropriate controls Biochemistry: Metabolism I 12/1/2009 Page 7 of 50 Evolution of Pathways: How have new pathways evolved? Add a step to an existing pathway Evolve a branch on an existing pathway Backward evolution Duplication of existing pathway to create related reactions Reversing an entire pathway Biochemistry: Metabolism I 12/1/2009 Page 8 of 50 Adding a step E1 E2 E3 E4 E5 ABCDEP Original pathway • When the organism makes lots of E, there’s good reason to evolve an enzyme E5 to make P from E. • This is how asn and gln pathways (from asp & glu) work Biochemistry: Metabolism I 12/1/2009 Page 9 of 50 Evolving a branch Original pathway: D E1 E2 A B C E3 X Fully evolved pathway: E3a D ABC E3b X Biochemistry: Metabolism I 12/1/2009 Page 10 of 50 Backward evolution Original system has lots of E P E gets depleted over time; Then D gets depleted; need to make it from D, so we evolve enzyme E4 to do that. need to make it from C, so we evolve E3 to do that And so on Biochemistry: Metabolism I 12/1/2009 Page 11 of 50 Duplicated pathways Homologous enzymes catalyze related reactions; this is how trp and his biosynthesis enzymes seem to have evolved Variant: recruit some enzymes from another pathway without duplicating the whole thing (example: ubiquitination) Biochemistry: Metabolism I 12/1/2009 Page 12 of 50 Reversing a pathway We’d like to think that lots of pathways are fully reversible Usually at least one step in any pathway is irreversible (Go’ < -15 kJ mol-1) Say CD is irreversible so E3 only works in the forward direction Then D + ATP C + ADP + Pi allows us to reverse that one step with help The other steps can be in common This is how glycolysis evolved from gluconeogenesis Biochemistry: Metabolism I 12/1/2009 Page 13 of 50 Oxidation-reduction reactions and Energy Oxidation-reduction reactions involve transfer of electrons, often along with other things Generally compounds with many C-H bonds are high in energy because the carbons can be oxidized (can lose electrons) Biochemistry: Metabolism I 12/1/2009 Page 14 of 50 Reduction potential Reduction potential is a measure of thermodynamic activity in the context of movement of electrons Described in terms of half-reactions Each half-reaction has an electrical potential, measured in volts, associated with it because we can (in principle) measure it in an electrochemical cell Biochemistry: Metabolism I 12/1/2009 Page 15 of 50 So what is voltage, anyway? Electrical potential is available energy per unit charge: 1 volt = 1 Joule per coulomb 1 coulomb = 6.24*1018 electrons Therefore energy is equal to the potential multiplied by the number of electrons Biochemistry: Metabolism I 12/1/2009 Page 16 of 50 Electrical potential and energy This can be expressed thus: Go’ = -nFEo’ n is the number of electrons transferred F = fancy way of writing # of Coulombs (which is how we measure charge) in a mole (which is how we calibrate our energies) = 96.48 kJ V-1mol-1 Biochemistry: Metabolism I 12/1/2009 Page 17 of 50 Oh yeah? Yes. 1 mole of electrons = 6.022 * 1023 e1 coulomb = 6.24*1018 e1 mole = 9.648*104 Coulomb 1 V = 1 J / Coulomb=10-3 kJ / Coulomb Therefore the energy per mole associated with one volt is 10-3 kJ / C * 9.648*104 C = 96.48 kJ Biochemistry: Metabolism I 12/1/2009 Page 18 of 50 What can we do with that? The relevant voltage is the difference in standard reduction potential between two half-reactions Eo’ = Eo’acceptor - Eo’donor Combined with free energy calc, we see Eo’ = (RT/nF ) lnKeq and E = Eo’ - (RT/nF ) ln [products]/[reactants] This is the Nernst equation Biochemistry: Metabolism I 12/1/2009 Page 19 of 50 Free energy from electron transfer We can examine tables of electrochemical half-reactions to get an idea of the yield or requirement for energy in redox reactions Example: NADH + (1/2)O2 + H+ -> NAD+ + H2O; We can break that up into half-reactions to determine the energies Biochemistry: Metabolism I 12/1/2009 Page 20 of 50 Half-reactions and energy NAD+ + 2H+ + 2e- NADH + H+, Eo’ = -0.32V (1/2)O2 + 2H+ + 2e- H2O, Eo’ = 0.82V Reverse the first reaction and add: NADH + (1/2)O2 + H+ NAD+ + H2O, Eo’ = 0.82+0.32V = 1.14 V. Go’ = -nFEo’ = -2*(96.48 kJ V-1mol-1)(1.14V) = -220 kJ mol-1; that’s a lot! Biochemistry: Metabolism I 12/1/2009 Page 21 of 50 Absorbance How to detect NAD reactions NAD+ 340 nm NADH NAD+ and NADH (and NADP+ and NADPH) Wavelength have extended aromatic systems But the nicotinamide ring absorbs strongly at 340 only in the reduced (NADH, NADPH) forms Spectrum is almost pH-independent, too! So we can monitor NAD and NADPdependent reactions by appearance or disappearance of absorption at 340 nm Biochemistry: Metabolism I 12/1/2009 Page 22 of 50 Classical metabolism studies Add substrate to a prep and look for intermediates and end products If substrate is radiolabeled (3H, 14C) it’s easier, but even nonradioactive isotopes can be used for mass spectrometry and NMR NMR on protons, 13C, 15N, 31P Reproduce reactions using isolated substrates and enzymes Biochemistry: Metabolism I 12/1/2009 Page 23 of 50 Next level of sophistication… Look at metabolite concentrations in intact cell or organism under relevant physiological conditions Note that Km is often ~ [S]. If that isn’t true, maybe you’re looking at the non-physiological substrate! Think about what’s really present in the cell. Biochemistry: Metabolism I 12/1/2009 Page 24 of 50 Mutations in single genes If we observe or create a mutation in a single gene of an organism, we can find out what the effects on viability and metabolism are In humans we can observe genetic diseases and tease out the defective gene and its protein or tRNA product Sometimes there are compensating enzyme systems that take over when one enzyme is dead or operating incorrectly Biochemistry: Metabolism I 12/1/2009 Page 25 of 50 Deliberate manipulations Bacteria and yeast: Irradiation or exposure to chemical mutagens Site-directed mutagenesis Higher organisms: We can delete or nullify some genes; thus knockout mice Introduce inhibitors to pathways and see what accumulates and what fails to be synthesized Biochemistry: Metabolism I 12/1/2009 Page 26 of 50 Nutrition Lots of nonsense, some sense on this subject Skepticism among MDs as to its relevance Fair view is that nutrition matters in many conditions, but it’s not the only determinant of health Biochemistry: Metabolism I 12/1/2009 Page 27 of 50 Macronutrients Proteins Carbohydrates Lipids Fiber Biochemistry: Metabolism I 12/1/2009 Page 28 of 50 Protein as food Source of essential amino acids Source of non-essential aa Fuel (often via interconversion to aketoacids and incorporation into TCA) All of the essential amino acids must be supplied in adequate quantities Biochemistry: Metabolism I 12/1/2009 Page 29 of 50 Which amino acids are essential? At one level, that’s an easy question to answer: they’re the ones for which we lack a biosynthetic pathway: KMTVLIFWH That shifts the question to: why have some of those pathways survived and not all? Answer: pathways that are complex or require more than ~30 ATP / aa are absent (except R,Y) Biochemistry: Metabolism I 12/1/2009 Page 30 of 50 The human list AA Asp Asn Lys Met Thr Ala Val Leu Ile moles ATP 21 22-24 50-51 44 31 20 39 47 55 essential? no no yes yes yes no yes yes yes Glu Gln 30 31 no no Biochemistry: Metabolism I AA moles ATP Arg 44 Pro 39 Ser 18 Gly 12 Cys 19 Phe 65 Tyr 62 Trp 78 His 42 12/1/2009 essential? no no no no no yes no* yes yes Page 31 of 50 Carbohydrates as food Generally recommended to be more than half of caloric intake Complex carbohydrates are hydrolyzed to glucose-1-P and stored as glycogen or interconverted into other metabolites Biochemistry: Metabolism I 12/1/2009 Page 32 of 50 Lipids as food You’ll see in 402 that the energy content of a lipid is ~ 2x that of carbohydrates simply because they’re more reduced They’re also more efficient food storage entities than carbs because they don’t require as much water around them Certain fatty acids are not synthesizable; by convention we don’t call those vitamins Biochemistry: Metabolism I 12/1/2009 Page 33 of 50 Vitamins Vitamins are necessary micronutrients A molecule that is a vitamin in one organism isn’t necessarily a vitamin in another E.coli can make all necessary metabolites given sources of water, nitrogen, and carbon Most eukaryotic chemoautotrophs find it more efficient to rely on diet to make complex metabolites We’ll discuss lipid vitamins first, then water-soluble vitamins Biochemistry: Metabolism I 12/1/2009 Page 34 of 50 Why wouldn’t organisms make everything? Complex metabolites require energy for synthesis Control of their synthesis is also metabolically expensive Cheaper in the long run to derive these nutrients from diet Biochemistry: Metabolism I 12/1/2009 Page 35 of 50 Vitamins: broad classifications Water-soluble vitamins Coenzymes or coenzyme precursors Non-coenzymic metabolites Fat-soluble vitamins Antioxidants Other lipidic vitamins Biochemistry: Metabolism I 12/1/2009 Page 36 of 50 Are all nutrients that we can’t synthesize considered vitamins? No: If it’s required in large quantities, it’s not a vitamin By convention, essential fatty acids like arachidonate aren’t considered vitamins Biochemistry: Metabolism I 12/1/2009 Page 37 of 50 Lipid vitamins Contain rings & long aliphatic sidechains At least one polar group in each Absorbed in intestine, carried via bile salts Hard to study Most are formally built from isoprene units, as are steroids Biochemistry: Metabolism I 12/1/2009 Page 38 of 50 Vitamin A (retinol) 3 forms varying in terminal polar group Involved in signaling and receptors b-carotene is nonpolar dimer Biochemistry: Metabolism I 12/1/2009 Page 39 of 50 Vitamin A deficiency Produces night blindness because the retina and cornea dry out Most common cause: nursing infants whose mothers have vitamin A deficiency in their diet Biochemistry: Metabolism I 12/1/2009 Page 40 of 50 Vitamin D Several related forms Hormones involved in Ca2+ regulation Figure courtesy Cyberlipid Biochemistry: Metabolism I (cholecalciferol) 12/1/2009 Page 41 of 50 Vitamin D deficiency Rickets in children: Bone disease, restlessness, slow growth One form of vitamin D is actually synthesizable from cholesterol given adequate sunlight; Therefore rickets is most common in densely settled urban environments Biochemistry: Metabolism I 12/1/2009 Page 42 of 50 Vitamin E (a-tocopherol) Phenol can undergo 1e- oxidation to moderately stable free radical Antioxidant activity prevents damage to fatty acids in membranes phenol Fig. Courtesy UIC pharmacy program Biochemistry: Metabolism I 12/1/2009 Page 43 of 50 Vitamin K (phylloquinone) Involved in synthesis of proteins involved in blood coagulation Reduced form involved as reducing agent in carboxylation reaction on glu sidechains Figure courtesy Cyberlipid Biochemistry: Metabolism I 12/1/2009 Page 44 of 50 Vitamin overdoses? QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. It’s difficult to overdose on water-soluble vitamins: excess is simply excreted Fat-soluble vitamins are stored in adipose tissue and can accumulate to high concentrations May be toxic even dietarily Therefore: don’t eat polar bear liver Biochemistry: Metabolism I 12/1/2009 Page 45 of 50 Ascorbate The only common water-soluble vitamin that is not a coenzyme or coenzyme precursor Vitamin in primates, some rodents Synthesizable in most other vertebrates Involved in collagen Reduced form acts as reducing agent during hydroxylation of collagen Deficiency gives rise to inadequate collagen scurvy Biochemistry: Metabolism I 12/1/2009 Page 46 of 50 PTM role of ascorbate Proline + O2 + a-ketoglutarate + ascorbate 4-hydroxyproline + succinate + CO2 + dehydroascorbate This is a post-translational modification that occurs to prolines within collagen The hydroxylated prolines help stabilize the collagen triple helix Hydroxylysine found in collagen too Biochemistry: Metabolism I 12/1/2009 Page 47 of 50 Dietary deficiency of ascorbate Primary sources of ascorbate are fruits, particularly citrus, and green vegetables Ascorbate deficiency’s first symptom involves collagen degradation, leading to scurvy Biochemistry: Metabolism I QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Image courtesy U.Cincinnati Medical School 12/1/2009 Page 48 of 50 Scurvy in history Shortage of green vegetables in sailors’ diets meant scurvy was rampant on shipboard until the 18th century Success of English navy over French 1760-1800 was partly due to the introduction of limes in English sailors’ diets 50 years before the French caught on Biochemistry: Metabolism I 12/1/2009 QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Page 49 of 50 Megadoses of ascorbate QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Linus Pauling (2-time Nobel laureate) became convinced late in his life that very high doses of ascorbate (> 1 g /day) were beneficial as a preventative His assertions were met with skepticism from the established medical community I would say the jury is still out! Biochemistry: Metabolism I 12/1/2009 Linus Pauling Image courtesy Oregon State U. Page 50 of 50