* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Biological Macromolecules
Survey
Document related concepts
Biosequestration wikipedia , lookup
Isotopic labeling wikipedia , lookup
Signal transduction wikipedia , lookup
Metalloprotein wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Photosynthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
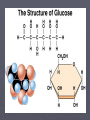
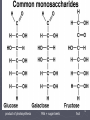
Protein structure prediction wikipedia , lookup
Biosynthesis wikipedia , lookup
Proteolysis wikipedia , lookup
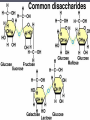
Transcript
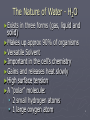
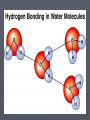
Water 1 The Nature of Water - H2O ►Exists in three forms (gas, liquid and solid) ►Makes up approx 90% of organisms ►Versatile Solvent ►Important in the cell’s chemistry ►Gains and releases heat slowly ►High surface tension ►A “polar” molecule: 2 small hydrogen atoms 1 large oxygen atom 2 3 2-3: Carbon Compounds • Compounds that contain CARBON are called organic. • Carbon has 4 electrons in outer shell. • Carbon can form covalent bonds with as many as 4 other atoms (elements). • Usually with C, H, O or N. • Example: CH4(methane) 4 ► 25 naturally occurring elements are essential for life. Carbon, hydrogen, oxygen and nitrogen (C, H, O, N) make up 96% of living matter. ► The remaining 4% is composed of seven elements (Ca, P, K, S, Na, Cl, Mg). Some elements, like iron (Fe) and iodine (I) may be required in very minute quantities and are called trace elements. 5 Biological Macromolecules 6 Macromolecules ► Large organic molecules. ► Also called POLYMERS. ► Made up of smaller “building blocks” called MONOMERS. ► The monomers in a polymer may be identical or they may be different. Some of the molecules that serve as monomers also have other functions on their own. ► Examples: 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic acids (DNA and RNA) 7 8 Carbon – the backbone of organic macromolecules! 9 Carbon – the backbone of organic macromolecules! Double bond Single bond 10 Polymerization ► The process of stringing together monomers to make polymers is called polymerization (or dehydration synthesis). ► Monomers are connected by a reaction in which two molecules are covalently bonded to each other through the loss of a water molecule. ► Each monomer contributes part of the water molecule that is lost: one molecule provides the – OH, while the other provides the hydrogen (–H). 11 Hydrolysis ► Polymers are disassembled to monomers by hydrolysis (from the Greek word hydro meaning “water” and lysis meaning “to split”.) ► In hydrolysis, polymers are split by water in a process that is essentially the reverse of dehydration synthesis. ► Bonds between monomers are broken by the addition of water molecules, a hydrogen from water attaching to one monomer and an –OH attaching to the adjacent monomer. 12 Animation ► Animation Hydrolysis of Dehydration Synthesis and 13 14 Carbohydrates 15 The Nature of Carbohydrates ► Recognized by the formula: (CH2O)n ► 1:2:1 ► Grouped into mono-, di-, and polysaccharides ► Important as: A “quick energy” nutrient - glucose Serve as a storage of energy - glycogen (animals) and starch (plants) Structural significance – chitin of Arthropods and fungus and cellulose of plants 16 17 product of photosynthesis Milk + sugar beets fruit 18 Common Disaccharides ► Sucrose (glucose + fructose) ►Found in plant like sugar cane, sugar beets ►“table sugar” ► Maltose (glucose + glucose) ►Found ► Lactose in germinating grain (glucose + galactose) ►Found in the milk of mammals 19 20 Polysaccharides • Cellulose – Structural component of plants (cell walls: lettuce, corn, some protists) • Starch – storage of energy in plants (bread, potatoes, rice) •Glycogen – storage of energy in animals (beef muscle) • Chitin – structural component of arthropods (roaches, crickets) 21 22 Lipids 23 The Nature of Lipids ► Lipids are one class of biological macromolecules that does not include polymers. ► The lipids are grouped together because they are not soluble in water. ► Lipids are a highly varied group in form and function. 24 ► Highest source of energy – that’s why our extra energy is stored as fat in fat tissue ► Also important as: structural components of cells (membranes) Chemical messengers - hormones (steroids) protective waxes (earwax, outer covering of insects) Protection against heat loss (insulation) 25 Examples of Lipids ► General term for compounds which are not soluble in water. ► Lipids are soluble in hydrophobic solvents. ► Remember: “stores the most energy” ► Examples: ►1. Fats ►2. Phospholipids ►3. Oils ►4. Waxes ►5. Steroid hormones ►6. Triglycerides 26 Fats ►(long-term energy storage ) ►Cushions organs ►insulation 27 ► Two types of molecules used to make a fat: ► Glycerol (three carbon molecule) ► Fatty acids (long hydrocarbon chain, usually 16-18 carbons atoms in length) that is hydrophobic (does not dissolve in water). 28 29 What Does Fat Look Like? 30 The Letter “E” Glycerol E Fatty Acids 31 32 Saturated vs. Unsaturated Fats ► Saturated Fats Solid at room temp. White in color Derived from animals No double bonds between carbons Fatty acids straight Examples are the hard fats (lard) BAD (unhealthy) ► Unsaturated Fats Liquid at room temp. Yellow in color Derived from plants Some double bonds between carbons Fatty acids crooked Examples include corn, canola, and olive oils BETTER (healthier) 33 34 Polyunsaturated Many carbon-carbon double bonds • Liquid at room temperature • Cooking oils: corn, sesame, peanut, canola, olive • BEST (healthiest) because they do not collect in your blood vessels and cause plaque. • 35 Phospholipids ► ► ► Phospholipids are a major component of cell membranes. The cell membrane regulates what enters and leaves the cell and provides protection and support. Phospholipids are similar to fats but they only have two fatty acid tails, instead of three, attached to a glycerol molecule. 36 ►A phospholipid has a hydrophilic head that wants to interact with water and two fatty acid tails that are hydrophobic and are repelled by water. 37 38 Steroids ► Steroids are lipids characterized by a carbon skeleton consisting of four fused rings. 39 ► One steroid, cholesterol, is a common component of animal cell membranes and is the precursor from which other steroids are synthesized. ► Too much cholesterol in the blood may contribute to heart disease. ► About 10% of people ages 12 to 19 have blood cholesterol levels, which put them at risk later in life for developing heart disease – the leading cause of death in the United States. 40 ► Hormones are chemicals released in one part of the body that travel through the bloodstream and affect the activities of cells in other parts of the body. ► They do this by binding to specific chemical receptors on target cells. If a cell does not have receptors, the hormone has no effect. 41 42 Proteins 43 The Nature of Proteins ►Elements: N, C, H, O ►Made of units called amino acids (only 20) ►Twenty Amino acids combine in different ways to make, perhaps, 100,000 different proteins! ►Amino acids link together by peptide bonds Makes polypeptides 44 Six functions of proteins: 1. 2. 3. 4. 5. 6. Storage: Transport: Regulatory: Movement: Structural: Enzymes: albumin (egg white) hemoglobin hormones muscles membranes, hair, nails, bones cellular reactions 45 46 47 Proteins (Polypeptides) ►Four levels of protein structure: A. Primary Structure (1°) B. Secondary Structure (2°) C. Tertiary Structure (3°) D. Quaternary Structure (4°) 48 A. Primary Structure (1°) ► Amino acids bonded together by peptide bonds. Amino Acids (aa) aa1 aa2 aa3 Peptide Bonds aa4 aa5 aa6 49 B. Secondary Structure (2°) ► 3-dimensional folding arrangement of a primary structure into coils and pleats held together by hydrogen bonds. ► Two examples: Alpha Helix Beta Pleated Sheet Hydrogen Bonds 50 51 C. Tertiary Structure (3°) ► Secondary structures bent and folded into a more complex 3-D arrangement. ► Bonds: H-bonds, ionic ► Called a “subunit”. Alpha Helix Beta Pleated Sheet 52 53 D. Quaternary Structure (4°) ► Composed of 2 or more “subunits”. ► Example: enzymes, hemoglobin 3° subunits 54 55 Enzymes ► ► ► ► ► ► Acts as catalysts Speed up a chemical reaction Lower activation energy Needed to start a reaction Specific Act on a substrate 56 Enzyme Action 57 Denaturation Denaturation of proteins involves the disruption and possible destruction of both the secondary and tertiary structures. Usually caused by: acids, bases, heat, alcohol 58 Nucleic Acids 59 60 The Nature of Nucleic Acids ► Two types: DNA and RNA ► Composed of units called nucleotides: Phosphate molecule 5 carbon sugar organic base (a purine or pyrimidine) ► May be double or single-stranded ► Single strands united by “base-pairing” 61 62 Organic (Nitrogenous) Bases 63 Importance of Nucleic Acids ► Insure genetic continuity from one cell generation to the next ► Directs the production of proteins at the ribosome “construction site” in the cytoplasm 64 65 66