* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download No Slide Title
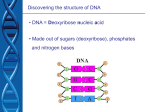
DNA sequencing wikipedia , lookup
Real-time polymerase chain reaction wikipedia , lookup
Agarose gel electrophoresis wikipedia , lookup
Zinc finger nuclease wikipedia , lookup
Protein–protein interaction wikipedia , lookup
DNA profiling wikipedia , lookup
Restriction enzyme wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Genomic library wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
SNP genotyping wikipedia , lookup
Transformation (genetics) wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Point mutation wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Community fingerprinting wikipedia , lookup
Biosynthesis wikipedia , lookup
Bisulfite sequencing wikipedia , lookup
Non-coding DNA wikipedia , lookup
Molecular cloning wikipedia , lookup
DNA supercoil wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
SEQUENCE SPECIFICITY DICTATED BY THE INTERPLAY BETWEEN DIRECT READOUT AND DNA FLEXIBILITY AT THE TATA BOX-BINDING PROTEIN - TATA BOX INTERFACE. Leonardo Pardo1, David Bosch1, Mercedes Campillo1, Nina Pastor2, and Harel Weinstein3 CENTRAL bps: TBP side chains: AA, AT ASN, THR AND GLY Forces: STACKING VAN DER WAALS Forces: H-BONDS -17.9 kcal/mol -17.8 kcal/mol N O1 Ade5 Gly216 N O1 2.32 a O2 2.08 O1 As n159 N2 Thy6 2.37 2.12 2.27 2.24 Gly125 N O1 Ade24 2.24 2.03 N 2.20 bps: GTAT: GATT: GTTT: GAAT: Thr124 Thr215 As n69 N2 Thy25 2.24 b O2 APPROACH: •We use quantum mechanical calculations to examine the interactions between TBP side chains and the basepair steps located at the most sequence conserved kink site (the 5’ kink: the first TA step, at the MP2 level), and at the only step recognized through the formation of H-bonds(the central basepair step, using DFT/BLYP3), to determine the role of direct readout in sequence discrimination. •We use Molecular Dynamics/Potential of Mean Force calculations with the AMBER 4.1 potential 43to estimate the free energy cost of transforming B- and A-DNA double stranded tetramers into the conformations found in high resolution crystal structures of TBP-DNA complexes, to determine the role of DNA bendability in TATA box selection by TBP. TATA SCE: 11.8 TAAA ATH: 8.1 From crystal structures and calculations: AT is always more distorted than AA (Kim Y. et al., 1993) INTRODUCTION: SCE complex O1' FINDINGS O1 Thy22 O2 2.17 O1 N a b TT/AA unfavorable due to steric clash in the major groove Thr82 Complex ii O1 As n117 N2 2.21 -17.9 kcal/mol As n27 2.11 Thy23 2.39 N 1.91 Gly174 1.84 2.49 Ade7 2.14 O1 O2 N2 N O1 Gly83 2.09 Thr173 Ade6 N Rise (Å) Complex i -17.9 kcal/mol Basepair F190 F207 P191 L205 ---------------------------------------------------------------------T2:A28 -4.4 -0.7 -1.6 A2:T28 -3.4 -0.7 -1.5 C2:G28 -3.0 -0.6 +1.5 ---------------------------------------------------------------------A3:T27 -7.0 -3.1 -1.6 T3:A27 -5.8 -3.5 -0.8 G3:C27 -7.1 -3.3 +32.9 TATA Thr82 (Kim Y. et al., 1993) N Gly83 2.61 Thy22 O2 O1 1.90 As n27 N2 2.16 Thy23 O2 TAAA TATA TAAA TATA 4 30 40 3 20 30 2 10 1 0 0 -10 TAAA 20 10 0 Calculated F for fitting TAAA into the TATA structure of SCE: 14.4 - 11.8 = 2.6 kcal/mol -14.6 kcal/mol 2.14 2.53 F transition F(TA) < F(AT) due to better H-bonds SCE complex: AT step •The TATA box-binding protein (TBP) binds specifically to 8 basepairs, using the minor groove surface of DNA. This mode of interaction is seen in all TBP-DNA complexes reported to date [1]. •The TATA box consensus sequence is TATA@A@N, where @ is A or T. •The minor groove of DNA is considered poor in information content, given the very similar placement of H-bond acceptors (T-O2 and A-N3) and hydrophobic sites (A-C2) in A•T and T•A basepairs. •The TBP-TATA box interface is mostly hydrophobic, with Leu, Pro, and Val side chains close to A-C2. •H-bonds are only found at the central basepair step of the TATA element, between Asn and Thr residues and the H-bond acceptors in the minor groove. •TBP bends and untwists the TATA box drastically. There are 45º kinks at the first and last basepair steps, and a 20º unwinding at the central basepair step. •The energetic cost of the various components of DNA distortions involved in the specific binding of TBP can be used to reveal the mechanisms underlying sequence specificity [2]. F 0.0 2.3 4.7 6.9 i nit ia f in l a TF A l I T F IA/ T T H IIB BP /T BP TA, AT, TT, AA, CG PHE, LEU AND PRO CENTRAL BP STEP: Free energy calculation of the A TA (ATH VS. SCE) transition for TAAA VS. TATA i ni t ia f in l a hu m a SC l nT E BP KINK bps: TBP side chains: 5’ KINK: Calculation of free energy differences for the B TA transition of various bps Twist (°) CENTRAL BP STEP: i nit ia f in l al TF IIA AT H TF / T IIB BP /T BP 5’ KINK: SEQUENCE DEPENDENT DNA FLEXIBILITY i ni t ia f in l a hu m a SC l nT E BP ENERGETICS OF DIRECT READOUT Roll (°) A common mechanism of DNA bending by minor groove-binding proteins is the insertion of protein side chains between basepair steps, exemplified in TBP/DNA complexes. At the first and last basepair steps of the TATA box, TBP kinks the DNA by inserting pairs of Phe side chains between the steps, and placing Leu and Pro side chains near the rim of the bases. QM calculations indicate that these side chains cannot discriminate between AT and TA basepairs. The sequence selectivity is due to the differential DNA flexibility of the basepair steps, as revealed by MD/PMF calculations, and to the ability of these steps to form H-bonds in the major groove. At the central basepair step of the TATA box, TBP markedly untwists this step, while engaging in hydrogen bonds with the bases and sugars. The H-bonds drive the conformational transition at this step, but are not capable of discriminating between AA and AT steps, as their strength is the same for both sequences. The calculated free energy cost for an equivalent conformational transition is found to be sequence dependent, being higher for AA steps than for AT steps. Consequently, AA steps have a smaller distortion in TBP/DNA complexes than AT steps. de Bioestadistica, Facultad de Medicina, Universidad Autonoma de Barcelona, 08193 Bellaterra, Spain; 2Facultad de Ciencias, UAEM, Av. Universidad 1001, Col. Chamilpa, 62210 Cuernavaca, Morelos, México; 3Department of Physiology and Biophysics, Mount Sinai School of Medicine, One Gustave L. Levy Place, New York NY 10029, U.S.A. i nit ia f in l a TF A l I T F IA/ T T H IIB BP /T BP 1Unidad i ni t ia f in l a hu m a SC l nT E BP ABSTRACT: c ATH complex: AA step (Kim J.L et al., 1994) •The interaction of Phe side chains cannot discriminate among these four A•T basepair steps, or between A•T and C•G basepairs. •Leu and Pro side chains clash against the N2 amino group in C•G basepairs, but cannot distinguish interactions with A•T from T•A basepairs. REFERENCES: [1]. Kim, Y. et al. (1993) Nature 365:520; Kim, J.L. et al. (1994) Nature Struct. Biol. 1:638; Nikolov, D.B. et al (1996) PNAS, USA 93:4862; Ju, Z.S. et al. (1996) J. Mol. Biol. 261:239; Patikoglou, G.A. et al. (1999) Genes Dev. 13:3217 [2]. Pastor, N. et al. (1997) Biophys. J.73:640; Pastor, N. et al. (1997) in Molecular Modeling of Nucleic Acids (ACS, Leontis, N.B. and SantaLucia Jr.,J., eds.) ) 268:329; Pardo, L., et al. (1998) Biophys. J. 74:2191; Pardo, L., et al. (2000) Biophys. J. in press; Pastor, N. and Weinstein, H. (2000) in Theoretical Biochemistry (Elsevier, Eriksson, L. ed.) in press. CONCLUSIONS: FINDINGS •The strength of the H-bonds made from Asn and Thr side chains to AA or AT basepair steps is practically the same. •Complex ii compensates the poorer interaction with DNA by improving the interaction within TBP. •Gly is important to stabilize the conformation of the Asn and Thr side chains. 1. DIRECT READOUT: 2. DNA DISTORTION: •Direct readout is not responsible for the selection of TA basepair steps at the 5’ kink. •TBP tolerates equally well AA and AT basepair steps at the central basepair step because the strength of the direct interactions to these two sequences is practically the same. 5’ KINK: •TA steps are the easiest to bend into the TA-DNA conformation, because of the interactions in the major groove in the final conformation: two good intra-strand H-bonds can be made and there are no clashes. CENTRAL BASEPAIR STEP: •AT steps are more distorted than AA steps in TBP-DNA complexes, because AT steps are easier to unwind and bend than AA steps.