* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 6- Diuretics
Discovery and development of direct Xa inhibitors wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Pharmacognosy wikipedia , lookup
Environmental impact of pharmaceuticals and personal care products wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Psychopharmacology wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
A diuretic is defined as a chemical that increases
the rate of urine formation.
By increasing the urine flow rate diuretic usage
leads to increase excretion of electrolytes
(especially Na+ & Cl-)and water from the body
without affecting protein,vitamins, glucose
amino acids reabsorption.
natriuretic
Na+
chloruretic
Cl-
saluretic
NaCl
kaliuretic
K+
bicarbonaturetic
HCO-3
Diuretics are used mainly in :
1. The relief of edema.
2. As adjuvant in the management of
hypertension.
3. Management of other disorders including;
congestive heart failure, chronic and acute
renal failure, glaucoma, hypercalcemia,
diabetes insipidus, and liver cirrhosis with
ascites.
Functions of the kidneys:
1.
2.
To maintain homostatic balance of electrolytes
and water.
To excrete water soluble end products of
metabolism.
So the kidneys accomplishes these functions
through the formation of urine by nephrons.
1.
2.
3.
4.
5.
Diuretics are acting at different sites in the nephron and
are classified as:
Carbonic anhydrase inhibitors acting at the proximal
convoluted tubule (site1 diuretics).
Loop diuretics acting at the Henle’s loop (site 2
diuretics).
Thiazides and thiazide-like diuretics acting at distal
convoluted tubule (site 3 diuretics).
Potassium-sparing diuretics acting at collecting tubule
(site 4 diuretics).
Osmotic diuretics; Act at proximal tubules, loop of
henle, collecting tubule.
Potency of a diuretic is related to the absolute
amount of drug (e.g mg/Kg)required to produce an
effect.
While efficacy relates to the maximum diuretic
effect (usually measured in terms of urine
volume/time or urine loss of Na+or NaCl/time).
Carbonic anhydrase is an enzyme containing Zinc.
It catalyzes the formation of carbonic acid (H2CO3 )
from CO2 and water.
CO2 + H2O
H2CO3
H+ + HCO3
Mechanism of action
These
compounds contain free sulfamoyl group
(SO2NH2) that is essential for activity.
The
SO2NH2 is isosteric with H2CO3, and is able to
occupy the receptor site of carbonic acid formation
and thus it must be non-substituted.
O
2
N
H
N N
5
S1
SO2NH2
N-[5-(Aminosulfonyl) 1,3,4-thiadiazol-2-yl]acetamide
Uses:
Acetazolamide used orally as tablets to reduce intraocular
pressure in the treatment of glaucoma.
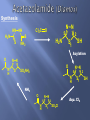
Synthesis
Cl2C
HN NH
H 2N
N N
O
5
S
S
2
H2N
NH2
SH
S
1
Acylation
O
N N
N
H
S
O
SO2NH2
N N
5
N
H
2
S
1
NH3
O
N N
5
N
H
Aqu. Cl2
2
S1
SO2Cl
SH
H3C
O
N N
2
N
5
S1
SO2NH2
N-[5-(Aminosulfonyl)-3-methyl- 1,3,4thiadiazol-2-(3H)-ylidene]acetamide
It is more potent derivative of acetazolamide due to more
lipophilic properties.
The increased lipophilicity is due to replacement of one of
the active hydrogen by methyl group. This permits a greater
penetration into occular fluids reducing intra-ocular
pressure.
It is used orally for treatment of glaucoma.
Cl
Cl
H2NO2S
SO2NH2
4,5-Dichloro-1,3-benzenedisulfonamide
Dichlorphenamide is a disulphonamide derivative
has mode of action and uses similar to
acetazolamide.
Cl
Cl
H2NO2S
SO2NH2
4,5-Dichloro-1,3-benzenedisulfonamide
Side Effects of CAEs:
1- Development of metabolic acidosis due to renal loss of
bicarbonate (system becomes more acidic& urine becomes
more alkaline).
2-Typical sulphonamide associated hypersensitivity reactions
e.g urticaria,drug fever,blood dyscrasias and interstitial
nephritis.
Cl
H2NO2S
NH2
SO2NH2
Chloraminophenamide
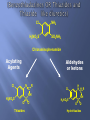
Acylating
Agents
Aldehydes
or ketons
Cl
N
H2NO2S
S
O
Thiazides
C
R
Cl
NH
O
H2NO2S
H
N H R
C
NH
S
O O
Hydrothiazides
The sodium transport system is responsible for the
reabsorption of Na+& Cl- in (DCT).
Inhibitors of the luminal membrane bound Na+/Clsystem include thiazide and thiazide like
diuretics(saluretic agents).
SAR:
1. Hydrogen at N-2 is the most acidic
because of the electron withdrawing
effect of the neighboring sulfone
group.
The acidic protons make possible
the formation of water soluble
sodium salts for I.V. administration.
SAR:
2.
Saturation of the double bond to
give 3,4 dihydro drvs give diuretic
from 3-10 times more active than
unsaturated drvs.
3.
Substitution at position 3 with
lipophilic group will affect potency
and duration of action, (CHCl2
,CH2C6H5, CH2SCH2CF3) results in
marked increase in potency&
duration of action
SAR:
4. Direct substitution at position 4,5 or 8
with alkyl group diminishes diuretic
activity.
5. Substitution at position 6 with
electron withdrawing gp (activating
gp) (Cl-, Br-, CF3-,NO2) is essential for
activity. Whereas substitution with
electron releasing gp(CH3 –or OCH3-)
results in marked reduction in
diuretic activity.
6. The Sulfamoyl gp at position 7 is a
prerequisite for diuretic activity.
Benzthiazide
(Exna)
Cl
H2NO2S
N
S
O
CH
NH
O
Cl
N
H2NO2S
S
O
C CH2 S CH2
NH
O
Duration of action From 6- Duration of action From 12-18 h
12h
The discovery that substitution of the sulfamoyl group
at position-1 in thiazide diuretics with another
electronegative group para to the activating group as
well as the opening of bicyclic hetero-system in
benzothiadiazines do not affect the diuretic activity.
That aids in the emergence of a group of diuretics
known as thiazide-like diuretics.
They are no longer benzothiadiazines, but site of action
and efficacy and side effects are similar to thiazide
diuretics.
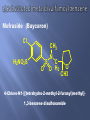
Mefruside (Baycaron)
Cl
H2NO2S
CH3
S
O
N
C
O H2
O
CH3
4-Chloro-N1-[(tetrahydro-2-methyl-2-furanyl)methyl]1,3-benzene disulfonamide
Clopamide (Brinaldix)
Cl
H2NO2S
H
C
N
CH3
N
O
H3C
3-(Aminosulfonyl)-4-chloro-N-(2,6-dimethyl-1piperidinyl)benzamide
1.
Hypersensitivity due to -SO2NH2 group
2.
Hypokalemia due to increase renal excretion of K +
3.
So potassium supplements are used (e.g. KCl, K
gluconate, K citrate), also use food rich with K+ as
banana, or used in combination with other diuretics
(potassium sparing diuretics)
1.
Administration of these diuretics with Non steroidal antiinflammatory drugs (NSAIDs)which inhibit prostaglandin
synthesis , can antagonize the diuretic effect of the former.
2.
Concurrent administration of these drugs with large doses of
Ca+2 containing substances may result in hypercalceamia
because of Ca+2 retaining properties of these diuretics.
3.
When these drugs are used with cardiac glycosides in
treatment of congestive heart failure , serious toxicity can
result if hypokalemia occurs
(Site 2 Diuretics) loop diuretics
High ceiling diuretics
Loop diuretics are very potent saluretic
agents. They are called so because they block
active Na+/Cl- transport at the thick ascending
limb of loop of henle ( 1Na+, 1 K+ , 2Cl- ) (MOA)
Their saluretic effect is much greater than that
produced by thiazides or other agents.
They are characterized by rapid onset (within
30 min)and short duration (6 h)
Classification
A. 5-Sulfamoyl-2-aminobenzoic Acid & 5sulfamoyl-3-aminobenzoic Acid derivatives.
B. Phenoxyacetic Acid derivatives.
C. 4-Amino-3-pyridinesulfonylureas
SAR:
NHR
NHR
H2NO2S
C
O
OH
5-Sulfamoyl-2-aminobenzoic acid
H2NO 2S
C
OH
O
5-Sulfamoyl-3-aminobenzoic acid
1- Substituents at position 1 must be acidic
COOH provides optimal diuretic activity
Other groups such as tetrazole may impart respectable diuretic
activity .
2- -SO2NH2 group at position 5 is prerequisite for activity.
3- Activating group at position 4 could be Cl-or CF3- in thiazides and
thiazide like diuretics or better with phenoxy, alkoxy, anilino,
benzyl or benzoyl
H
N
Cl
H2NO2S
O
COOH
4-Chloro-N-furfuryl5- sulfamoyl anthranilic acid
Synthesis:
Cl
Cl
Cl
Cl
ClSO 3H
OH
NH3
OH
H2NO2S
O
O
Cl
H2NO2S
H
N
COOH
NH2
O
O
H
N
(CH2)3 CH3
O
H2NO2S
COOH
3-(Butylamino)4- phenoxy-5- sulfamoylbenzoic
acid
Cl- is replaced by Phenoxy group
The short duration of action is similar to that of
furosemide but Bumetanide is 50 times more
potent than furosemide.
Uses:
Treatment of pulmonary edema associated with
congestive heart failure
Side Effects
1- -SO2NH2 group hypersensitivity
2- Ototoxicity So care must be noticed when used
with aminoglycosides.
3-NSAIDs may blunt the natriuresis produced by
loop diuretics in patients with preexisting
impaired renal function who are on diuretic
therapy NSAIDs may increase the risk of renal
failure
Ethacrynic acid (Edecrin)
Cl
O
H3CH2C
C
H
H
4
3
Cl
2
1
O CH2 COOH
2-(2,3-dichloro-4-(2methylenebutanoyl)phenoxy)acetic acid
Mechanism of action:
Inhibition of sulfhydryl-containing enzymes involved
in solute reabsorption
Side Effects:
1- greater incidence of ototoxicity
2- produce more serious GIT effects (GIT heamorrhage)
than sulfamoyl containing loop diuretics.
3- The effect with NSAIDs the same as with furesemide
& bumetanide
Torsemide
N
H3C
N
H
CH3
O
O2S
N
H
N
H
CH3
1- isopropyl-3-{[4-(3-methylphenylamino)pyridine]-3sulfonyl}urea
Torsemide contain sulfonyl urea group instead of sulfonamide
group in furosemide &bumetanide
N
N
N
N
Site & MOA: It interferes with
Pteridine
the process of cationic exchange in the
distal tubule. It blocks re-absorption of Na+ and blocks excretion of K+ .
The net result is increased NaCl excretion in the urine and almost no K+
execretion
Example: Triametrene.
H2N
N
N
NH2
N
N
NH2
Triametrine
Side Effects:
1- Hyperkaalemia so K+ levels should be
regulated and checked& K+ supplements
should be controlled
2- form renal stones
Amiloride HCl (Midamor)
O
Cl
NH
N
N
H
H 2N
N
NH 2
NH2
6-Chloro-3,5-diamino-N-(aminoiminomethyl)pyrazine-2- carboxamide.
SAR:
1- Optimum activity is obtained when position 6 is
substituted with Cl 2- NH2 group at positions 3,5 are unsubstituted.
The adrenal cortex secretes a potent mineralocorticoid
called aldosterone which promotes :
– Na+& Cl- reabsorption (salt retention)
_ K+ excretion
This effect is 3000 times more potent than
hydrocortisone
A substance that antagonizes the effects of
aldosterone could be a good diuretic drug. It is
called Spironolactone
Spironolactone
is
a
competitive
antagonist
to
the
mineralocorticoids such as aldosterone.
The
mineralocorticoid
receptor
is
an
intracellular
protein in nature that can bind aldosterone.
Spironolactone binds to the receptor and competitively
inhibits aldosterone binding the the receptor .
The inability of aldosterone to bind to its receptor
prevents reabsorption of Na+& Cl-and
associated water.
The most important site of these receptors is in
the late distal tubule and collecting system
Side Effects:
1-Hyperkalemia and mild metabolic acidosis,
therefore patients taking spironolactone should
be warned not to take K+ supplements.
2- caution must be considered when
administering spironolactone with either
(ACE) inhibitors & β-adrenergic blockers.
A) Osmotic diuretics
Osmotic diuretics are low-molecular-weight
compounds that are not extensively
metabolized and are passively filtered through
Bowman’s Capsules into the renal tubules.
Once in the renal tubules they have limited
reabsorption. They form a hypertonic solution
and cause water to pass from the body into the
tubules, producing a diuretic effect.
Polyols such as mannitol, sorbitol and isosorbed provide this effect.
Mannitol (osmitrol) and sorbitol are used intravenously in solutions of 550%.
Isosorbide is basically a bicyclic form of sorbitol used orally to cause a
reduction in intra-ocular pressure.
CH 2OH
HO
H
HO
H
H
OH
H
OH
CH 2OH
Mannitol
H
OH
O
H
OH
Isosorbide
Uses:
1- Diagnosis & prophylaxis of acute renal failure
2- decrease intraocular pressure
3- To promote urinary excretion of toxic
substances
B) Theophylline
Theophylline, xanthine derivatives that
promote a weak diuresis by stimulation of
cardiac function and by direct action on the
nephron.
Used infrequently as diuretic, but diuresis may be
observed as side effect when it is used as
bronchodilator.