* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 7
Photoredox catalysis wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Electrolysis of water wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Asymmetric induction wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Drug discovery wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Acid–base reaction wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Process chemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Electrochemistry wikipedia , lookup
Atomic theory wikipedia , lookup
Chemical bond wikipedia , lookup
Catalytic reforming wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Metalloprotein wikipedia , lookup
History of chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Abiogenesis wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Marcus theory wikipedia , lookup
Isotopic labeling wikipedia , lookup
George S. Hammond wikipedia , lookup
Biochemistry wikipedia , lookup
Photosynthesis wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Molecular dynamics wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
History of molecular theory wikipedia , lookup
Click chemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Transition state theory wikipedia , lookup
Organic chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
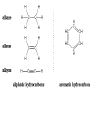
Chapter 7 Chemistry in Action Chemical Reactions • A chemical reaction is a chemical change resulting from a collision of atoms or molecules. • The original substances are reactants • The substances produced by the reaction are called products for example: carbon can collide with oxygen and make carbon dioxide Chemical Equation: C + 2 O = CO2 Conservation of Mass In any chemical reaction, atoms are conserved… That is, the same number of atoms used in the reaction is the same number of atoms in the products. This is conservation of mass (or matter)…Matter is not created or destroyed, it just changes form. Energy in Chemical Reactions • Energy Changes: Some reactions “need” energy to occur and therefore absorb heat during a reaction – these are endothermic reactions Reactants + energy → products Some reactions “give” energy in a reaction and therefore give off heat – these are exothermic reactions Reactant → products + energy Energy in Chemical Reactions Heat and other natural processes in a system always tend toward less usable energy and greater disorder… This is known as the second law of thermodynamics When you eat something, only about 55% of energy is actually used…the rest is converted into heat and “lost” to your body This energy cannot be used again…. Energy in Chemical Reactions Entropy: The amount of decay or disorder in a system According to the 2nd law of thermodynamics, entropy always tends to increase; a decrease in entropy in one place requires a greater increase of entropy somewhere else. Factors Affecting Rates of Reactions • Much of practical chemistry involves identifying the reaction characteristics of different substances so that reactions between them may be conducted safely and efficiently in the laboratory and industry! Factors Affecting Rates of Reactions 1. Temperature – an increase in temperature will usually speed up a chemical reaction. This is because the rising temperature causes the atoms to move more quickly. Factors Affecting Rates of Reactions 2. Concentration – Increased concentration increases the rate of reaction. This is because, the more atoms that are squeezed into a limited space, the more likely they are to react with each other. Factors Affecting Rates of Reactions 3. Surface Area – increasing the surface area of the reactants increases the speed of the reaction. This is because there is more area over which the molecules can collide and react. Example: Grinding a substance into a powder allows it to burn more easily! Factors Affecting Rates of Reactions 4. Catalysts – A catalyst is a substance that alters the rate of a chemical reaction without being permanently changed in the reaction. Catalysts change the activation energy of the reaction!!! Your body has thousands of catalysts called enzymes that God created to keep your body running smoothly! Types of Chemical Reactions There are four basic categories of chemical reactions: 1. Combination Reactions 2. Decomposition Reactions 3. Single-replacement Reactions 4. Double-replacement Reactions Combination Reactions • Chemical reactions which combine two or more substances to form a third substance. • Also called synthesis reactions. A + B → AB Example: C + O2 → CO2 Decomposition Reactions • Complex compounds break down into different substances. AB → A + B Example: CaCO3 → CaO + CO2 Single-Replacement Reactions • One element is replaced by another element. A + BC → AC + B Example: 2 NaBr + Cl2 → 2 NaCl + Br2 Double-Replacement Reactions • Two compounds swap ingredients. • Two compounds react to form two new compounds. AC + BD → AD + BC Example: AgNO3 + NaCl → NaNO3 + AgCl Reversing a Reaction • Some chemical reactions are reversible. • These reactions can be made to go back the other way. Salts • A salt is a general term that refers to an ionic compound. • When salts dissolve in water, they “dissociate” (physically separate) in component ions. • So in water, NaCl is really Na+ ions and Cl- ions. Acids and Bases Acids produce hydrogen ions (H+) in solution… A acid has a pH of 1 to 6 Bases produce hyroxide ions (OH-) in solution A base has a pH of 8 to 14 Something neutral has a pH of 7…examples are milk and salts Human blood is a weak base…it has a pH of 7.3 pH scale The pH Scale Organic Chemistry • Organic chemistry has to do with any compound containing carbon. • Organic compounds include: fuels, foodstuff, paper products, cosmetics, plastics, soaps, fabrics, and paints. Carbon is Special! • More compounds are formed with carbon than all other elements combined! • Why? 1. Carbon forms four covalent bonds. 2. Carbon can bond to form several different shapes. 1. Rings 2. Chains 3. Three dimensional shapes Carbon is Special! 3. Carbon atoms may form single, double, or triple bonds. 4. Carbon may form single and double bonds with the atoms of many other elements. 5. Carbon may form compounds that contain different structural arrangements and combinations with the same molecular formula. isomers: carbon compounds having the same molecular formula but different structural formulas. Hydrocarbons • Compounds that contain only hydrogen and carbon are called hydrocarbons. • Most industrial compounds are hydrocarbons. Naming Hydrocarbons • Hydrocarbons are named according to the number of carbons in the molecules and the arrangement. • The first part is the prefix…it is based on the number of carbon atoms. Naming Hydrocarbons • Alkanes: Hydrocarbons with one bond are called alkanes. – Example: propane, petroleum jelly • Alkenes: Hydrocarbons with one or more double bond are called alkenes. – Example: beta carotene (vitamin A), olive oil Naming Hydrocarbons • Alkynes: Hydrocarbons with one or more triple bond are classified as alkynes. – Example: used in manufacture of plastics • Cyclic hydrocarbons: ring shaped carbon molecules. – Example: cyclohexane • Aromatic hydrocarbons: ring shaped, but there are no double or triple bonds. – Example: benzene, TNT, mothballs Substituted Hydrocarbons • Hydrocarbons that have one or more hydrogen atoms replaced by different atoms or groups of atoms are called substituted hydrocarbons. • The substituted group is called a functional group. • Substituted hydrocarbons include: haloalkanes, alcohols, caboxylic acid, and esters. Haloalkanes • The hydrogen atoms are replaced by one of the halogens (chlorine, fluorine, bromine, iodine, or astatine) • Example: freon and methane Alcohols • One or more of the hydrogens are replaced by an OH group. • Example: methanol made from methane. Carboxylic Acids • One or more hydrogen atom replaced by a carboxyl group (COOH) • Example: acetic acid Esters • The insertion of an ester group. • An ester group is –COO• Most have pleasant odors and are used for perfumes and flavor enhancers. Soaps • Soaps are a useful organic compound in that it can dissolve non-polar substances. Polymers • Polymers are synthetic materials and have been useful in plastics and synthetic fibers. • They are long molecular chains that link many smaller molecules together. • Common polymers are polyesters, polystyrene, and teflon.