* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Genome Editing of a CArG Element in the Mouse Genome
Frameshift mutation wikipedia , lookup
Zinc finger nuclease wikipedia , lookup
Genomic imprinting wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
Gene nomenclature wikipedia , lookup
Non-coding DNA wikipedia , lookup
Human genome wikipedia , lookup
Genome (book) wikipedia , lookup
Saethre–Chotzen syndrome wikipedia , lookup
Oncogenomics wikipedia , lookup
Transposable element wikipedia , lookup
Gene desert wikipedia , lookup
Gene expression profiling wikipedia , lookup
Genetic engineering wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Public health genomics wikipedia , lookup
Genomic library wikipedia , lookup
Gene therapy wikipedia , lookup
Gene expression programming wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Point mutation wikipedia , lookup
Helitron (biology) wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Microevolution wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Genome evolution wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
History of genetic engineering wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Designer baby wikipedia , lookup
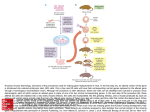
Editorial Genome Editing of a CArG Element in the Mouse Genome Establishes its Role in Gene Expression Kiran Musunuru I n the February issue, Han et al1 present the use of a novel clustered regularly interspaced short palindromic repeat (CRISPR)-CRISPR-associated 9 (Cas9) method to inactivate the calponin-1 gene involved in smooth muscle contraction, by introducing a knockin mutation to cleanly disrupt a CC(A/T)6GG (CArG) element in the first intron of the gene. Previous work by the group had suggested a critical role of the CArG element in calponin-1 gene expression in both humans and mice.2 Here, the authors injected the components of CRISPR-Cas9—an mRNA for the Cas9 endonuclease, a guide RNA containing the 20-nucleotide sequence matching the sequence of the CArG element, and a 135-nucleotide single-strand DNA oligonucleotide overlapping the CArG element and containing the desired knockin mutation—into the cytoplasm of fertilized mouse eggs (Figure). This resulted in stable incorporation of the CArG knockin mutation into the genome in some of the eggs. After implantation of the resulting blastocysts into surrogate mothers, the injected eggs yielded founder mice in 3 weeks. Remarkably, 3 of 18 founder mice (17%) carried the desired knockin mutation on ≥1 chromosome, with 1 founder having the mutation on both chromosomes. The founder mice were successfully bred, with germline transmission of the knockin mutation. With calponin-1 being a smooth muscle cell–restricted gene, the authors examined smooth muscle tissues from wild-type, heterozygous, and homozygous mice and found almost complete suppression of calponin-1 gene expression in the homozygous mice. This simple yet elegant experiment, performed within a time frame of a few months, unequivocally establishes the critical role of the CArG element in calponin-1 gene expression in smooth muscle tissues. See accompanying article on page 312 in the February 2015 issue To fully appreciate the game-changing nature of CRISPRCas9, consider a similar study performed in the pregenomeediting era. Khromov et al3 engineered a knockin mouse in which a 30-nucleotide fragment containing an AT-rich sequence and a CArG element was deleted to suppress telokin gene expression in smooth muscle tissues. This entailed a long series of steps. First, a double-strand targeting vector containing 6-kb and 3-kb homology arms flanking an antibiotic resistance cassette (replacing the 30-nucleotide fragment and flanked by loxP sequences) was produced. Second, the targeting vector was electroporated into mouse embryonic stem cells, which were selected with antibiotic and then expanded. Third, mouse embryonic stem cell clones were screened for correct insertion of the antibiotic resistance cassette into the telokin locus. Fourth, embryonic stem cells were injected into blastocysts and implanted into surrogate mothers to yield chimeric mice. Fifth, the chimeric mice were bred to obtain mice that had inherited the mutant allele through the germline. Sixth, as part of the breeding, male mice expressing Cre recombinase in the germline were used to remove the antibiotic resistance cassette. Finally, the floxed alleles were bred to homozygosity to yield the final mice for study. A conservative estimate of the amount of time required to carry out all these steps is ≥1 year and probably ≈2 years; yet, despite all this effort, the end result was a mutant allele in which the 30-nucleotide CArG-bearing fragment was replaced with a 34-nucleotide loxP sequence, effective but crude. In contrast, Han et al1 were able to carry out their study in just a few months while creating a more subtle mutant allele in which several nucleotides were substituted to impair the CArG element, with no need for antibiotic resistance, the Cre-loxP system, and so on. The tremendous use of CRISPR-Cas9 in generating knockout and knockin mice was initially demonstrated by Rudolf Jaenisch’s group4,5 and has since been validated with its ability to correct pathogenic mutations in mouse embryos6,7 and to generate knockout and knockin mutations in a wide variety of model organisms, most impressively in monkeys.8 The work by Han et al1 represents the first case in which CRISPR-Cas9 has been used to manipulate smooth muscle gene expression by editing a key noncoding regulatory element and can easily be extended to rapidly and efficiently interrogate the function of any number of noncoding regulatory elements throughout the mouse genome. This successful demonstration of the method should have a long-time impact not only in the field of smooth muscle biology but also in many other fields of biomedical research. Disclosures From the Department of Stem Cell and Regenerative Biology, Harvard University, Cambridge, MA; and Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA. Correspondence to Kiran Musunuru, MD, PhD, MPH, Harvard University, 7 Divinity Ave, Cambridge, MA 02138. E-mail [email protected] (Arterioscler Thromb Vasc Biol. 2015;35:496-497. DOI: 10.1161/ATVBAHA.115.305175.) © 2015 American Heart Association, Inc. Arterioscler Thromb Vasc Biol is available at http://atvb.ahajournals.org DOI: 10.1161/ATVBAHA.115.305175 None. References 1. Han Y, Slivano OJ, Christie CK, Cheng AW, Miano JM. CRISPR-Cas9 genome editing of a single regulatory element nearly abolishes target gene expression in mice––brief report. Arterioscler Thromb Vasc Biol. 2015;35:312–315. doi: 10.1161/ATVBAHA.114.305017. 2.Long X, Slivano OJ, Cowan SL, Georger MA, Lee TH, Miano JM. Smooth muscle calponin: an unconventional CArG-dependent gene that antagonizes neointimal formation. Arterioscler Thromb Vasc Biol. 2011;31:2172–2180. doi: 10.1161/ATVBAHA.111.232785. Downloaded from http://atvb.ahajournals.org/ at UNIV OF ROCHESTER on April 14, 2015 496 Musunuru CArG Element in the Mouse Genome 497 AQ3 Figure. Accelerated generation of knockin mice with clustered regularly interspaced short palindromic repeat (CRISPR)CRISPR-associated 9 (Cas9). PCR indicates polymerase chain reaction. 3. Khromov AS, Wang H, Choudhury N, McDuffie M, Herring BP, Nakamoto R, Owens GK, Somlyo AP, Somlyo AV. Smooth muscle of telokin-deficient mice exhibits increased sensitivity to Ca2+ and decreased cGMP-induced relaxation. Proc Natl Acad Sci U S A. 2006;103:2440–2445. doi: 10.1073/ pnas.0508566103. 4. Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. 5. Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/ Cas-mediated genome engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. 6. Wu Y, Liang D, Wang Y, Bai M, Tang W, Bao S, Yan Z, Li D, Li J. Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell Stem Cell. 2013;13:659–662. doi: 10.1016/j.stem.2013.10.016. 7. Long C, McAnally JR, Shelton JM, Mireault AA, Bassel-Duby R, Olson EN. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science. 2014;345:1184–1188. doi: 10.1126/ science.1254445. 8. Niu Y, Shen B, Cui Y, et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156:836–843. doi: 10.1016/j.cell.2014.01.027. Key Words: gene expression transgenic mice ■ gene knockout ■ Downloaded from http://atvb.ahajournals.org/ at UNIV OF ROCHESTER on April 14, 2015 ■ smooth muscle Genome Editing of a CArG Element in the Mouse Genome Establishes its Role in Gene Expression Kiran Musunuru Arterioscler Thromb Vasc Biol. 2015;35:496-497 doi: 10.1161/ATVBAHA.115.305175 Arteriosclerosis, Thrombosis, and Vascular Biology is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2015 American Heart Association, Inc. All rights reserved. Print ISSN: 1079-5642. Online ISSN: 1524-4636 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://atvb.ahajournals.org/content/35/3/496 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Arteriosclerosis, Thrombosis, and Vascular Biology can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Arteriosclerosis, Thrombosis, and Vascular Biology is online at: http://atvb.ahajournals.org//subscriptions/ Downloaded from http://atvb.ahajournals.org/ at UNIV OF ROCHESTER on April 14, 2015