* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chem vocab quiz definitons
Photoredox catalysis wikipedia , lookup
Metallic bonding wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Electrochemistry wikipedia , lookup
Isotopic labeling wikipedia , lookup
Chemical potential wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Click chemistry wikipedia , lookup
Elementary particle wikipedia , lookup
Drug discovery wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Marcus theory wikipedia , lookup
Electronegativity wikipedia , lookup
Chemical bond wikipedia , lookup
Chemical reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
History of molecular theory wikipedia , lookup
Electron configuration wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Transition state theory wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Periodic table wikipedia , lookup
Stoichiometry wikipedia , lookup
Chemical element wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
History of chemistry wikipedia , lookup
Atomic nucleus wikipedia , lookup
Extended periodic table wikipedia , lookup
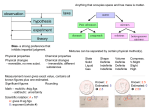
Name: _______________ class: ___________________ date:_________ Chemistry vocab quiz16 answers Atomic/Molecular structure definitions. Matter anything that has volume and mass. Proton is a positively charged particles that are found in the nucleus, and has the mass of 1 atomic unit (amu). Neutron is an electrically neutral particles that are found in the nucleus, and has a mass of 1 amu. Electron is a negatively charged sub atomic particle found in an area outside the nucleus of the atom. Atomic number is the number of protons in the nucleus. It is the number of protons that identifies the element. Atomic mass is number that indicates the sum of the protons and neutrons in the nucleus. This number is an average of the masses of all isotopes of that element and therefore a decimal. Valence electrons are the electrons in the outer shell and control the elements reactivity. Molecule is the smallest particle of a compound that has all the properties of the compound. Compound is a pure substance that is made of two or more elements chemically bound together. Pure substances are both elements and compounds Isotopes are various atoms of an element (ex Boron; 115 B, 105 B, 95 B) that differ in the number of neutrons.. Ion is an atom that is no longer electrically neutral because it has gained or lost an electron. Physical Change/ Characteristics Liquid is the state of matter that is described as having a definite volume but an indefinite shape. Gas is the state of matter that is described as having no definite shape, or volume. Solid is the state of matter that is described as having a definite shape and volume. Viscosity is a property of liquids that describe their resistance or ability to flow. Crystal is a solids in which the particles form a regular 3 dimensional pattern, common to ionic compounds. Condensation is the change of state from gas to liquid. Sublimation the change of state from solid directly to gas. Solubility is a measure of how well a substance can dissolve in another substance at a given temperature. Solute is the part of the solution that is dissolved by another substance. Solvent is the part of the solution that dissolves other substances. Chemical Equations and Formulas Subscript is a number placed to the lower right of the elemental symbol indicating the number of atoms of the element in the compound. Coefficient a number placed before an element or compound in a chemical equation that multiplies the entire molecule that follows. Periodic Table Period is a row (horizontal) in the Periodic Table of Elements. Metal is the most common classification of elements. A group or family is a combination of elements that are shown as a column in the Periodic Table of Elements. These elements share the same number of valence electrons and react with the same elements. Metalloids are the elements located along the staircase or zig-zag line on the Periodic Table of Elements. Noble Gasses is the name of the column of elements that don’t react with other elements. Chemical Reactions and Equations . Law of Conservation of Mass or Matter is the principle that the amount of matter in a normal chemical reaction doesn’t change so the total mass of the reactants must equal the total mass of the products. Physical Change is a change in shape or form. Law of Conservation of Energy is the principle that the total amount energy stays the same during a chemical reaction. Ionic Bond is a chemical bond formed as a result of the attraction of oppositely charged ions. Activation Energy is the minimum amount of energy required to start a reaction. Exothermic Reaction is a chemical reaction that liberates energy to the environment. Endothermic Reaction is a chemical reaction the absorbs energy. Reactants are the elements and compounds that go into a reaction. These appear on the left side of the arrow that is read as yields. Products are the elements and compounds that come out of a reaction. These appear on the right side of the equation. Yields is how the arrow in the chemical equation is read. Temperature is measure of the average kinetic energy of the atoms and molecules.