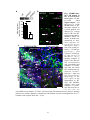
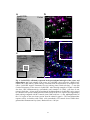
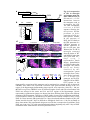
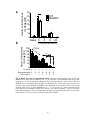
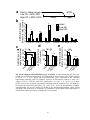
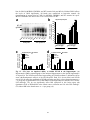
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download GLUCOCORTICOIDS INCREASE CNS INFLAMMATION
Neuroeconomics wikipedia , lookup
Signal transduction wikipedia , lookup
Haemodynamic response wikipedia , lookup
Synaptogenesis wikipedia , lookup
Nervous system network models wikipedia , lookup
Subventricular zone wikipedia , lookup
Development of the nervous system wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Synaptic gating wikipedia , lookup
Neuroanatomy wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Environmental enrichment wikipedia , lookup
Optogenetics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Endocannabinoid system wikipedia , lookup
GLUCOCORTICOIDS INCREASE CNS INFLAMMATION, WORSENING ACUTE NEUROLOGICAL INJURY A DISSERTATION SUBMITTED TO THE DEPARTMENT OF BIOLOGY AND THE COMMITTEE ON GRADUATE STUDIES OF STANFORD UNIVERSITY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Shawn Fletcher Sorrells June 2011 © 2011 by Shawn Fletcher Sorrells. All Rights Reserved. Re-distributed by Stanford University under license with the author. This work is licensed under a Creative Commons AttributionNoncommercial 3.0 United States License. http://creativecommons.org/licenses/by-nc/3.0/us/ This dissertation is online at: http://purl.stanford.edu/ww382cb9419 ii I certify that I have read this dissertation and that, in my opinion, it is fully adequate in scope and quality as a dissertation for the degree of Doctor of Philosophy. Robert Sapolsky, Primary Adviser I certify that I have read this dissertation and that, in my opinion, it is fully adequate in scope and quality as a dissertation for the degree of Doctor of Philosophy. Ben Barres I certify that I have read this dissertation and that, in my opinion, it is fully adequate in scope and quality as a dissertation for the degree of Doctor of Philosophy. Firdaus Dhabhar I certify that I have read this dissertation and that, in my opinion, it is fully adequate in scope and quality as a dissertation for the degree of Doctor of Philosophy. Anton Wyss-Coray Approved for the Stanford University Committee on Graduate Studies. Patricia J. Gumport, Vice Provost Graduate Education This signature page was generated electronically upon submission of this dissertation in electronic format. An original signed hard copy of the signature page is on file in University Archives. iii Abstract Glucocorticoids (GCs) are stress hormones that are well-known for their potent and pleiotropic anti-inflammatory effects. In the injured CNS, their anti-inflammatory properties could be particularly beneficial due to the often detrimental effects of excessive inflammation in the brain. In more recent years, however, it has become clear that GCs do not always decrease inflammation and can even augment aspects of the immune response. The research presented in this doctoral dissertation sought to determine the impact of this increased inflammatory response in the CNS using animal models of excitotoxicity and hypoxia/ischemia. Specifically, both exogenous GC treatments and the endogenously released GCs post-injury were found to increase immune cell activation (both in phenotype and in p65 nuclear translocation) in both rat and mouse models of excitotoxic hippocampal neuron death and in a mouse MCAO stroke model. These increased inflammatory responses are likely to be mediated by an unexpected GCsuppression of several anti-inflammatory cytokines including CX3CL1 and CD22 and failure of GCs to activate some of their normal anti-inflammatory targets like IkBa, IL-1ra, and MKP-1. Furthermore, these GC-augmented inflammatory responses are necessary for GCs to make more neurons die from either of these injuries. Taken together, this work demonstrates that cellular inflammation is not kept in check by GCs in the forebrain; instead, GCs worsen hippocampal and cortical neuron death, at least in part, by increasing the neurotoxicity of CNS inflammation. iv Acknowledgements: The work presented in this dissertation would never have been possible without the care and devotion of the following people: Robert Sapolsky, Ben Barres, Firdaus Dhabhar, Tony WyssCoray, Theo Palmer, Javier Caso, Carolina Munhoz, Nathan Manley, Angela Lee, Bonnie Chien, Kevin Tran, Caroline Hu, and all of the members of the Sapolsky lab. The following individuals provided instrumental and lively discussions about the research along the way: Norman Ruby, Dritan Agalliu, Tyler Cutforth, Brenda Kusler, Marta Perez, Andy Evans, Eric Hoopfer, Takaki Komiyama, Dev Manoli, and Joe Lipsick. In addition, many of the most interesting findings discovered in these investigations were a direct result of the supreme generosity of Dr. Louis Muglia who freely provided floxed-GR mice. Drs. Sam Okret and Ben Barres also kindly provided mouse lines for these investigations. Finally, I would especially like to thank my brother Trevor and my parents Mark and Nancy Sorrells for their love and support over these past years. v Table of Contents Chapter 1: Background and Literature Review 1 1.1 Introduction 1 1.2 Glucocorticoid regulation of immunity outside the nervous system 3 1.2.1 Classic anti-Inflammatory effects of glucocorticoids 3 1.2.2 The pro-inflammatory effects of stress and GCs outside the nervous system 5 1.3 Mechanisms of neuron death and inflammation 10 1.3.1 Neuron death 10 1.3.2 The inflammatory response to neuron death 11 1.4 Glucocorticoid regulation of immunity in the nervous system 12 1.4.1 Normal glucocorticoid effects in the brain 12 1.4.2 Glucocorticoids endanger neurons 12 1.4.3 Glucocorticoid effects on inflammatory cells in the CNS 14 1.4.4 Glucocorticoid effects on inflammatory cytokines in the CNS 15 1.4.5 Glucocorticoid effects on inflammatory transcription factors 16 1.5 Overview of experimental hypotheses 16 1.5.1 Primary hypotheses tested 17 1.5.2 Secondary hypotheses tested 18 Chapter 2: GC Activation of CNS Immune Responses 20 2.1 Experimental model 20 2.1.1 MGRKO mice have GR deleted in myeloid cells 20 2.1.2 FBGRKO mice have GR deleted in forebrain neurons 21 2.1.3 MGROV and FBGROV mice have inducible overexpression of GR in myeloid cells or neurons 22 2.1.4 GC levels in experimental system 22 2.1.5 Injury models 23 2.2 Results 24 vi 2.2.1 GR signaling in myeloid cells increases immune cell activation and recruitment 24 2.2.2 GCs increase immune cell activation in the injured rat hippocampus 26 2.3 Discussion 27 2.3.1 Endogenous and exogenous GCs affect different aspects of cellular inflammation post-injury 27 2.3.2 GCs increase immune cell activation in the injured hippocampus 28 2.4 Conclusions 29 Chapter 3: Mechanisms of GC-Augmented Immune Responses 31 3.1 Results 31 3.1.1 Hypothesis (i): GCs increase pro-inflammatory cytokines and chemokines 31 3.1.2 Hypothesis (ii): GCs decrease anti-inflammatory signaling molecules 32 3.1.3 Hypothesis (iii) GCs increase NF-kB activity 34 3.2 Discussion 35 3.2.1 Hypothesis (i): GCs do not increase pro-inflammatory cytokines and chemokines 35 3.2.2 Hypothesis (ii): GCs decrease anti-inflammatory signaling molecules 37 3.2.3 Hypothesis (iii) GCs increase NF-kB activity 38 3.3 Conclusions 40 Chapter 4: GC-Endangerment of Neurons Requires GC-Augmented Inflammation 41 4.1 Results 41 4.1.1 GC-endangerment requires GC-augmented inflammation 41 4.1.2 GR signaling in myeloid cells endangers neurons in mice 41 4.1.3 GR signaling affects BDNF levels differently in the hippocampus and cortex 42 4.1.4 Endothelial cell GR is also detrimental to neuron survival post-injury 43 4.2 Discussion 44 4.2.1 GC effects on inflammation contribute to GC endangerment of neurons 44 4.2.2 GC endangerment requires myeloid cell GR, not neuron GR 45 4.2.3 Endogenous GCs are also detrimental to neuron survival, but less so 46 vii 4.2.4 GR signaling has cell-autonomous effects on BDNF levels 47 4.2.5 GR signaling in endothelial cells also endangers neurons during MCAO 48 4.3 Conclusions 50 Chapter 5: General Discussion and Conclusions 51 5.1 Primary hypotheses tested and interpretations 51 5.1.1 GCs augment inflammation 51 5.1.2 GCs suppress anti-inflammatory cues and increase NF-kB activation 55 5.1.3 GC-augmented inflammation is necessary for neuron death 57 5.2 Secondary hypotheses tested and interpretations 57 5.2.1 Timing and duration of GC exposure 57 5.2.2 Synthetic versus endogenous GCs 59 5.2.3 Basal/permissive levels of GCs versus stress levels 60 5.2.4 Regional differences in GC effects in the brain 61 5.2.5 Does GC-augmented inflammation and neuron endangerment occur in mice? 62 5.3 Summary and Conclusions 62 Materials and Methods 66 Figures 72 Tables 93 References 95 viii List of illustrations Fig. 1. MGRKO mice have GR deleted in the parenchymal microglia of the cortex and hippocampus. 72 Fig. 2. LysM-CRE is efficiently expressed in the parenchymal microglia of the cortex and hippocampus. 73 Fig. 3. FBGRKO mice have GR deleted in neurons of the cortex and hippocampus. 74 Fig. 4. Overexpression of GR in microglia and neurons using the same CRE-drivers. 75 Fig. 5. Blood GC levels in experimental model. 76 Fig. 6. GCs enhance cellular inflammation and can both enhance and suppress recruitment. 77 Fig. 7. Use of flow cytometry to identify CNS leukocytes. GR overexpression does not alter cell-recruitment. 78 Fig. 8. Minocycline reverses GC-augmented immune cell activation in the hippocampus. 79 Fig. 9. GCs do not increase cellular inflammation by enhancing chemokine production. 80 Fig. 10. GCs suppress anti-inflammatory gene expression. 81 Fig. 11. GCs affect CX3CL1 levels oppositely in the hippocampus post-KA and cortex post-MCAO. 82 Fig. 12. GCs suppress pro-inflammatory cytokines and are necessary for COX-2 expression. 83 Fig. 13. GCs have an impaired ability to inhibit NF-kB in the hippocampus. 84 Fig. 14. GCs increase p65 nuclear localization. 85 Fig. 15. GC-augmented inflammation is necessary for GC-endangerment. 86 Fig. 16. GC-endangerment requires GR signaling in myeloid cells but not neurons. 87 Fig. 17. GR overexpression has minimal effects on neuron death. 88 Fig. 18. GCs suppress BDNF levels in the hippocampus and cortex in a cell-specific manner. 89 Fig. 19. Endothelial cell KO and OV mice have higher GR levels in endothelial cells. 90 Fig. 20: Endothelial cell GR signaling also worsens infarct post-MCAO. 91 Fig. 21. Summary of endogenous GC and exogenous GC effects during different injuries. 92 ix List of tables: Table 1. Some experimental conditions where GCs have been found to increase inflammation. 93 Table 2. Summary of parameters that impact how GCs affect inflammatory responses. x 94 Chapter 1: Background and Literature Review 1.1 Introduction We are born with the ability to respond to stress, and this is fortuitous because the act of being born is the first of many stressors that we will experience during life. In humans and other vertebrates, stressful circumstances elicit a series of physiological changes that constitute the stress response. This begins within seconds with the release of the catecholamines of the sympathetic nervous system (epinephrine and norepinephrine). In the ensuing minutes, the hypothalamic-pituitary-adrenal (HPA) axis is activated. CRF produced by the hypothalamus acts on the pituitary to activate the release of ACTH into circulation, resulting in the release of the adrenal steroid glucocorticoids (GCs). The role of GCs is largely to modulate and control the stress response over a longer timeframe in the minutes to hours following a stressor. This response is conducted largely on a genomic level and accordingly the actions of GCs are numerous and varied (1). Our understanding of the stress response aptly began during the Great Depression. In 1935, Walter Cannon described the extraordinary flexibility of the body in its ability to respond to stress, or “accidents of existence” (2). In 1936, Hans Selye described the “general adaptation syndrome”, activated by an organism in order to overcome various challenges (3). Cannon called the stress-induced increases in cardiac output the “fight or flight” response, and he had realized the importance of adrenal hormones in this response as early as 1924 (4). “Fight or flight,” as described by Cannon, reflects the actions of both catecholamines and GCs (although the latter were unknown until the work of Selye) to immediately increase cardiovascular output and blood flow to the brain and skeletal muscles. This is predominately mediated by catecholamines, although GCs potentiate their effects (1). Both hormones mobilize energy stores from adipose and hepatic cells, ensuring a supply of energy to exercising muscle. At the same time, GCs decrease less immediately essential activities such as feeding, digestion, growth and reproduction (1). Thus, two separate hormone systems conduct divergent effects over time on a variety of physiological parameters as an organism responds to stress. 1 Stress also has potent effects on the immune system, and indeed one of Selye’s original observations was that chronic GC exposure caused the thymus to atrophy (5). Over the next decades, GCs began to be widely utilized in clinical practice for their particularly potent immunosuppressive properties. As research began to reveal the mechanisms underlying GCmediated immunosuppression, it also became apparent that stress and stress hormones can also have immune-activating properties. Several different research groups have identified parameters that lead to stress increasing immune responses (6-10). Some of the most important of these factors are (1) the type of stress hormone (2) timing of hormone exposure (3) duration of hormone exposure, and (4) hormone concentration. Like GCs, catecholamines can increase or decrease immune responses, but these effects are not considered further here. GC effects also vary depending on whether they are synthetic or endogenous, but the effects of physiological levels of endogenous GCs were selected as the primary focus for this work. The most immunestimulating effects are caused by acute exposure to GCs and these augmented immune responses can be beneficial to injury recovery (11). In contrast, chronic exposure to GCs is more frequently detrimental and immunosuppressive (6). This explanation fits well into an adaptive understanding of the immune system during the stress response where it needs to be mobilized initially, but must be kept under control in the long term (9). In the injured CNS, the consequences of excessive or prolonged inflammation are particularly dire, so GCs are widely used in clinical neurology. Additionally, CNS injury is a stressor itself so endogenous GCs are also present. Given that GCs can sometimes increase inflammation, it is essential to know how they affect CNS inflammatory responses to direct their proper use. The next sections review the existing literature about how stress and GCs affect immune responses, beginning with their well-studied anti-inflammatory effects and then discussing the conditions that lead to increased immune responses in the periphery. Finally, the small but growing body of literature studying GC-augmented inflammatory responses in the CNS is discussed. 2 1.2 Glucocorticoid regulation of immunity outside the nervous system 1.2.1 Classic anti-Inflammatory effects of glucocorticoids Hans Selye in the 1930’s obtained the first evidence for GCs being anti-inflammatory, noting that sustained stress or GC exposure caused involution of the thymus. This was such a dramatic and reliable effect that it was long used as a bioassay for circulating GC concentrations, before the development of radio-immunoassays. The stress-induced thymic involution described by Selye was subsequently shown to be caused by GR-mediated apoptosis in immature T cells (12). In the wake of their establishment as profoundly anti-inflammatory, synthetic GCs came to be used in many domains of clinical pharmacology. Work since then has examined GC-induced immunosuppression on far more reductive levels than the involution of an organ. On the cellular level, GCs can inhibit both the innate and adaptive immune responses. GCs inhibit lymphocyte proliferation, induce apoptosis in basophils and eosinophils, and cause a redistribution of T-cells (13). GCs also cause dendritic cells (DCs), the antigen-presenting cells of the adaptive immune system, to maintain an immature state (13). In addition, GCs can induce T-cell apoptosis and a shift in the population of T cells from mediating cellular immunity with a Th1 phenotype to mediating humoral immunity with a Th2 phenotype (1). On the molecular level, GCs limit the capacity of DCs to interact with immature T-cells by inhibiting MHC II expression. Moreover, GCs inhibit or reduce the expression of a variety of chemoattractants and pro-inflammatory cytokines (e.g. IL-1β, IL-6, TNF-α), while enhancing the expression of anti-inflammatory cytokines (e.g. IL-10, TGF-beta) (1). The expression of other pro-inflammatory molecules such as inducible COX-2 and prostaglandins is also inhibited by GCs. The inhibition of cytokines and other pro- inflammatory molecules has been demonstrated at the levels of both protein and mRNA synthesis. In addition, GCs enhance the production of decoy receptors and antagonist molecules such as IL-1R2 and IL-1Ra, resulting in decreased IL-1 signaling (although the latter is disputed) (14). GCs exert these varied effects through well-established mechanisms of steroid hormone 3 signaling. Upon cellular entry, GCs bind to either the Type I, mineralocorticoid receptor (MR), or the Type II glucocorticoid receptor (GR). MR has a much higher affinity (0.5 nM) for GCs than does GR (5.0 nM), and this difference is a large part of the reason for concentration-dependent differences in GC activity (15). At basal GC levels, GCs predominantly bind to MR and only slightly occupy GR. In the early stage of a stressor, MR becomes saturated and the occupancy of GR is increased. It is not until a sustained, major stressor that GR occupancy is saturated. Thus, MR is responsible for much of the effects of basal and low-stress levels of GCs (i.e. the permissive effects), whereas GR largely mediates the effects of high stress GC levels. This, combined with the fact that MR and GR signaling often have opposing outcomes, results in a Ushaped curve of GC action where intermediate (basal to low-stress) GC concentrations have the opposite effect of no GCs or high-stress concentrations (1, 10). Both MR and GR belong to a large class of nuclear receptors which homodimerize and translocate to the nucleus after ligand binding. There, both MR and GR bind to the glucocorticoid response element (GRE) consensus DNA sequence, and modulate the transcriptional activity of downstream genes in a similar manner. Because both MR and GR can bind to the same GRE, their often opposite effects arise from their different patterns of protein-protein interactions with other components of the transcriptional machinery. Unlike MR, GR directly interferes with the transcriptional activity of the pro-inflammatory transcription factors nuclear factor kappa B (NFκB) and activating protein 1 (AP-1), thus decreasing expression of the inflammatory genes they would normally activate. The result is an extensive decrease in inflammatory mediators (reviewed in (14)). Interestingly, reports of rapid non-genomic signaling by these nuclear hormone receptors suggest a mechanism by which GCs might be able to mediate temporally distinct effects (16, 17). Such non-genomic effects likely occur via protein–protein interactions between the GR and other cytosolic or nuclear factors; however, there are also more enigmatic membrane-bound nuclear hormone receptors that are also thought to mediate some of the rapid signaling effects of GCs. As discussed, GCs do not always decrease inflammation. The next section describes how 4 stress was found to have immune-enhancing effects and the realization that GCs are actually responsible for some of these increases. 1.2.2 The pro-inflammatory effects of stress and GCs outside the nervous system Stress has been assumed to be anti-inflammatory due to the well-understood antiinflammatory actions of GCs; however, this is not necessarily the case. In particular, acute stress just before an inflammatory challenge often has the adaptive effect of enhancing the immune response, whereas chronic stress has the role of keeping the immune response in check by suppressing it (6). For example, one type of the delayed type hypersensitivity (DTH) cellmediated immune response is exacerbated by acute stress but suppressed by chronic stress (18). Moreover, peripheral and central nervous system (CNS) levels of pro-inflammatory cytokines such as IL-1β are increased following an acute stressor in both rats (19) and humans (20). Additionally, the activity of NFκB and expression of NFκB target genes increase following acute stress in human peripheral blood mononuclear cells (21) and rat cortex (22). These studies all concluded that acute stress enhanced the response to a subsequent immune challenge. The timing of the relationship between stressor and immune challenge is critical, in that acute stress after an inflammatory challenge decreases expression of IL-1β, TNF-α, IL-6, and other proinflammatory cytokines (23). Given the widespread anti-inflammatory actions of GCs, it seems most probable that some other aspect of the stress response is responsible for immune enhancement by acute stress. Some evidence implicates catecholamine release by the sympathetic nervous system (SNS). For example, acute stress enhances the recruitment of leukocytes to sites of immune activation (24). Furthermore, stress-induced increases in NFκB signaling were modeled in human monocyte THP-1 cells after stimulation with norepinephrine, suggesting that SNS activation may be responsible for stress-induced cytokine increases (21). Injection of alpha and beta adrenoceptor antagonists block the stress-mediated increase in IL-1β levels, and adrenoceptor agonists are sufficient to substitute for stress (25). Because the actions of catecholamines are exerted on a shorter timescale than those of GCs, these results fit with the picture of acute and early stages of 5 stress as pro-inflammatory. For further review of the pro-inflammatory actions of stress, see (6, 8). While many pro-inflammatory effects of acute stress can be attributed to catecholamines, GCs are also involved. Several recent microarray studies examined the effects of the high-affinity GR agonist dexamethasone on human peripheral blood mononuclear cells and found that amidst expected anti-inflammatory changes in gene expression, there were many pro-inflammatory systems such as chemokines, complement proteins, and cytokines with increased expression (26, 27). Several more in depth investigations have identified other situations where GCs increase peripheral inflammatory responses and those are discussed next. The instances discussed are by no means comprehensive; for instance, the stimulatory effects of GCs on the acute phase response to inflammation are not discussed. See (1, 6, 28, 29) for further review of GC effects on peripheral inflammation. As noted, GCs have profound negative effects on the numbers of circulating leukocytes and can induce apoptosis (30). However, a closer look reveals that neutrophil proliferation and survival are enhanced by GCs (31, 32) and that the large-scale depletion of other leukocytes may actually reflect extravasation to a site of injury, rather than cell death (24, 33, 34). The depletion of circulating leukocytes by GCs has been described metaphorically as a deployment of immune soldiers to the battlefront rather than to the barracks (35). Acute stress-enhanced migration of leukocytes from the bloodstream to wound sites can be mimicked by either a physiological dose of corticosterone or a GR agonist (but not an MR agonist), and is blocked by adrenalectomy (ADX) or inhibition of GC synthesis (34). Notably, chronic stress also fails to have the same effects on leukocyte redistribution and instead decreases baseline leukocyte numbers as well as the magnitude of acute stress effects on leukocyte redistribution (33). These findings implicate GR occupancy at GC levels typical of an acute stressor as both necessary and sufficient to mediate enhancement of this particular immune response. Acute stress levels of GCs have also been shown to enhance a model of peripheral inflammation. Acute stress-induced enhancement of the DTH immune response is blocked by 6 adrenalectomy, which removes both GCs and epinephrine and is restored by supplementation with corticosterone (the GC specific to rodents) levels that replicate the early stages of the acute stress-response (33). This restoration is achieved to a greater extent by the replacement of corticosterone than epinephrine (18). This surprising result may be due in part to the GCenhanced leukocyte migration following acute stress. In contrast, chronic exposure to corticosterone or acute exposure to dexamethasone does not have the same effect and is antiinflammatory (18, 36). It is an important caveat that in some models of DTH and for some types of stressors, GCs and catecholamines have not been seen to play an important role in stressmediated augmentation (36, 37). The differences between the effects of acute and chronic stress on immune function described by Dhabhar and McEwen (33) cannot be explained by the concentration-dependent effects of MR and GR signaling. As noted, the pro-inflammatory migration of leukocytes to wound cites is GR- rather than MR-mediated. Thus, the most meaningful way of understanding the dichotomy between the pro- and anti-inflammatory actions is not so much the contrast between MR and GR occupancy, as the contrast between acute and major/prolonged stressresponses. Presumably, these differences are manifested on the molecular level through the duration and extent of GR occupancy. GCs can have permissive and suppressive actions through mechanisms other than activation of the opposing effects of MR and GR signaling. While GCs reduce the expression of most pro-inflammatory cytokines, they often simultaneously increase the expression of the corresponding cytokine receptor (38). This means that at low GC levels, cytokines are in abundance, but their receptors are not and signaling is low. Conversely, at high GC levels, receptors are in abundance, but cytokines are suppressed so signaling is low. Thus, cytokine signaling is minimal at extreme GC concentrations and peaks at an intermediate GC concentration (39). This observation has been shown to have functional importance in the proliferative effect of IL-2 signaling on T cells. GCs decrease IL-2 production, but they expedite expression of the IL-2 receptor. The result is that acute stress-levels of GCs enhance T cell 7 proliferation by accelerating the ability of IL-2 to signal through its receptor (40, 41). Notably, exposure to chronic, high levels of corticosterone prevented this effect (42). The applicability of this model to other cytokines and their receptors remains to be investigated. Weigers and Reul have suggested that these effects of GCs on cytokine signaling might serve to optimize the course of inflammation (38). Although GCs inhibit many pro-inflammatory cytokines, they increase expression of at least one potent pro-inflammatory mediator. The macrophage migration inhibitory factor (MIF) was one of the earliest discovered pro-inflammatory cytokines. Its release from intracellular pools in macrophages and T cells results in the production of nitric oxide and TNF-α in an autocrine stimulatory manner (43). MIF also has been implicated in DTH, as MIF-blocking antibodies attenuate the DTH immune response (44). In humans, circadian plasma levels of GCs correlate with a circadian pattern of MIF concentrations (45). Moreover, basal concentrations of GCs increase MIF production in T cells and macrophages. Strikingly, physiologically relevant concentrations of MIF can completely overcome the anti-inflammatory effects of GCs on other aspects of inflammation in LPS stimulated monocytes (46). Furthermore, in a high dose in vivo LPS challenge where GC treatment reduces mortality, concomitant treatment with MIF negates the protection conferred by GCs. Taken together, these findings suggest a physiological role for MIF in countering the anti-inflammatory effects of GCs (47). GC enhanced MIF production is dose-dependent and fits the model that low-levels of GCs are pro-inflammatory and high doses are anti-inflammatory. Low physiological doses of cortisol, the species typical GC of humans and most primates, stimulate MIF secretion in murine monocytes, while high doses suppress its production (46). Surprisingly, dexamethasone was as stimulatory as cortisol at low doses and became anti-inflammatory at approximately the same dose. Given the striking difference in GR binding affinity between these two GCs, this result suggests that these effects of GCs on MIF are not necessarily controlled by the degree to which GR is bound. For the most part, the actions of GCs on MIF fit the model that low doses suppress, and high doses enhance inflammation. 8 The timing of GC exposure relative to an immune challenge has additional importance in determining their action. When GCs are administered prior to an inflammatory challenge, they have actually been found to augment the subsequent cytokine response. Treatment with GCs twelve hours prior to LPS and IFN gamma stimulation increases NF-κB signaling and IL-6, TNFα, and nitrite production in a murine macrophage cell line (48). In a similar manner, exposure to GCs twelve hours before LPS stimulation increases plasma levels of TNF-α and IL-6 in humans (49). This enhanced response is not necessarily due to preparative cytokine receptor upregulation because similar increases are not observed when GCs are given six hours prior to inflammatory challenge. Finally, acute stress twenty-four hours prior to LPS increases plasma levels of TNF-α, IL-6, and IL-1β in the rat; however the role of GCs in this outcome was not tested (50). In each instance of GC pre-treatment, GCs had been removed from the media at the time of the inflammatory stimulation in vitro, and in the in vivo model, GC levels had returned to baseline at the time of LPS exposure. Thus, these effects are not due to the concentration of GCs at the time of the inflammatory response. Instead, Maier et al described stress as having a preparative “priming” effect on the subsequent inflammatory response to an immune challenge (50-52). It is clear that much work remains to be done to fully understand what governs when GCs are pro- or anti-inflammatory in the periphery. As proposed by (6-10), GC type and concentration, time course of exposure, and the activation state of the immune system are all significant. Several trends emerge among the diverse GC enhancing effects on inflammation: low-doses, acuteexposure, and GC presence prior to inflammation all tend to augment it, whereas high-doses, chronic-exposure, and GC presence after inflammation tend to suppress it. The evidence suggests that in the context of an acute stressor and immune challenge, GCs play a complex role in orchestrating an optimal and timely response that maintains control over the potentially damaging effects of an over-extended immune response. Before considering how GCs affect the CNS inflammatory response, the unique nature of CNS injury is discussed. Unlike in the periphery, the CNS has considerably lower tolerance for inflammation and the consequences can be devastating to neurons when it goes unchecked. 9 Inflammatory responses in the injured CNS are also regulated in different in several ways than those in the periphery due to the cellular architecture of the CNS. The next section discusses two common types of acute neurological injury, namely excitotoxicity and hypoxia/ischemia. These neurological injuries can cause extensive neuron death and inflammation, and more of one often leads to more of the other. 1.3 Mechanisms of neuron death and inflammation 1.3.1 Neuron death While there are many different types of acute traumatic injuries to the CNS (e.g. stroke, TBI, seizure, toxin exposure), many of the fundamental things that lead to neuron death are the same. At the heart of most of these traumatic injuries is an energy crisis. This can be an active process as during seizure where neurons fire in excess ultimately depleting their energy reserve, or it can be a more passive process, as during stroke where the lack of blood flow to the injury site interrupts the supply of energy. In addition, seizure is frequently a secondary consequence of TBI and stroke. The depletion of energy has multiple consequences for neurons. First, it disrupts the active process of glutamate re-uptake from the synapse, leading to heightened extracellular glutamate levels. This stimulates post-synaptic neurons to continue to fire, amplifying the energy crisis. Second, it disrupts the active process of Ca2+ sequestration in response to neuronal depolarization. When neurons fire, intracellular calcium levels rise as Ca2+ flows in via voltagegated Ca2+ channels and NMDA receptors. The excessive Ca2+ levels lead to over-activation of intracellular processes that ultimately result in the increased production of reactive oxygen species (ROS), protein mis-folding, cytoskeletal breakdown and membrane oxidation. ROS scavenging is also energy dependent, further worsening the problem. One last-ditch option that cells can choose if they still have enough energy to begin the process is a programmed cell-death sequence. This process, called apoptosis, is frequently initiated in neurons that were far enough away from the first traumatic events that they survived, but were further compromised during secondary injury processes. Apoptosis is a good option for 10 a cell that cannot sufficiently recover because it helps to contain the release of neurotoxic and immune-activating factors and debris that occurs during necrotic cell death. Although costly, it protects neighboring neurons by giving cells a non-toxic way to dismantle their components into self-contained vesicles. Ultimately, phagocytes will clean up these remaining compartments, neutralizing the threat. The effect is proportional in expense and benefit to getting rid of nuclear warheads by dismantling them and dealing with the waste properly instead of detonating them. 1.3.2 The inflammatory response to neuron death These processes rapidly activate the immune response and local microglia extend their processes and activate pro-inflammatory gene transcriptional programs. Microglia are activated by perturbations in the extracellular environment like the presence or concentration of metabolic substrates that are normally intracellular (e.g. ATP (53)), thus signifying an imbalance in neuronal homeostasis. Microglia release pro-inflammatory cytokines such as IL-1beta, IL-6, and TNFalpha and chemokines such as MCP-1 and CINC-1. These molecules increase microglial proliferation in an autocrine fashion, and signaling to other immune cells to recruit them to the injury site. Whether this inflammatory response is beneficial or detrimental to the neurons that survive the initial insult is important for understanding how to appropriately treat neurological insults. At least some attributes of the inflammation appear to be beneficial because TNF-α receptor knockout mice have increased lesion size following neuronal insult (54). In contrast, TNF-alpha and IL-1beta have been seen to increase neuron death (55), and at least IL-1beta is implicated in worsening the extracellular accumulation of glutamate and intracellular calcium influx during seizure (56). Other cytokines such as IL-6 and IL-10 have anti-inflammatory or neuroprotective properties (55). The cellular inflammatory response is similarly divided. Astrocyte proliferation may have protective effects by isolating and containing the inflammatory response to the immediate injury site. Supporting this, inhibiting astrocyte proliferation worsened neuron loss in focal brain injury models (57). In contrast, peripheral granulocytes that are recruited release additional pro- 11 inflammatory cytokines, proteases, and oxygen radicals and these factors are responsible for much of the damage that occurs (58). The most neurotoxic peripheral cells are granulocytes and lymphocytes, whereas monocyte recruitment has been attributed a protective role (59). Broadly, the early stages of a moderate inflammatory response in the injured brain can be beneficial, whereas more extensive inflammation can adversely affect surviving neurons (60). The next section describes how GCs manipulate these different aspects of the immune response to CNS injury and what is currently known about the consequences of this for neuron death. 1.4 Glucocorticoid regulation of immunity in the nervous system 1.4.1 Normative glucocorticoid effects in the brain Glucocorticoids have an enormous and varied array of effects upon the brain. In particular, hippocampal cells express high levels of MR and GR and are a primary target of GC actions. Such actions include various salutary outcomes mediated by MR occupancy and/or the small increase in GR occupancy seen in the early phase of the stress-response. These basal and permissive effects include prevention of neuron death in the dentate gyrus, enhancement of synaptic plasticity, and facilitation of hippocampal-dependent cognition (1). However, GC actions in the hippocampus show the classic inverse-U pattern, in that heightened and prolonged GC exposure has an array of deleterious effects, mediated by high GR occupancy. These include impaired cognition and synaptic plasticity, inhibition of neurogenesis, atrophy of dendritic arbors, and a reduction in spine density (61). 1.4.2 Glucocorticoids endanger neurons The adverse consequences of prolonged elevation of GC concentrations are particularly dramatic when coincident with neuronal injury. An extensive literature now demonstrates that chronic stress and GCs, signaling through GR, can compromise the ability of neurons in the hippocampus, cortex and striatum to survive a variety of neurological insults. Endogenous GCs are protective during the injury caused by intraparenchymal LPS-injection (62), but are detrimental during excitotoxicity (63). Exogenous GC treatment is more clearly deleterious and 12 can exacerbate neuron death from a wide variety of injury types including hypoxia-ischemia (64), seizure (63), hypoglycemia (65), oxygen radical generators (66), and β-amyloid (67). These “endangering” actions are exacerbated by the fact that many of these neurological insults stimulate additional GC secretion. A fair amount is now understood about the mechanisms underlying this endangerment. Specifically, GCs inhibit glucose uptake throughout these brain structures (68), an effect that is not sufficient to be energetically disruptive by itself, but it exacerbates the declines in ATP concentrations and mitochondrial potential caused by these insults. As a result, affected neurons and astrocytes have less energy available for the costly tasks of high-affinity re-uptake of the potentially excitotoxic glutamate from the synapse (69, 70). In addition, affected neurons are compromised in their ability to sequester and/or extrude free cytosolic calcium from post-synaptic neurons (71), and to contain and quench oxygen radicals (66), all of which imperil neurons (72, 73). In light of their anti-inflammatory properties, this suggests that chronic GCs should exert two opposing effects in the injured brain. On the one hand, GCs directly compromise the ability of various neuron types to survive a number of neurological insults, augmenting damage. In contrast, by decreasing the extensive inflammation caused by these insults, GCs should decrease damage (and in support of this, non-steroidal anti-inflammatory agents that blunt inflammation are neuroprotective (74, 75)). This raises the issue of whether the endangering GC effect upon neurons, or their protective inhibition of extensive inflammation is more dominant. The fact that exposure to high stress concentrations of GCs augments the extent of neurotoxicity and inflammation in response to these insults in vivo suggests that the endangering effects prevail (72). These findings come as somewhat of a surprise given the anti-inflammatory potential of chronic GCs. However, it turns out that at least part of the explanation is that the antiinflammatory effects of chronic GCs in the brain are actually less consistent and potent than in the periphery. 13 1.4.3 Glucocorticoid effects on inflammatory cells in the CNS GCs are effective at reducing certain types of inflammatory cytotoxicity in the brain. For example, treatment with dexamethasone decreases iNOS-mediated toxicity of microglia (76). Direct infusion of LPS into the prefrontal cortex causes necrotic cell death accompanied by an extensive inflammatory response and is commonly used as a model for inflammatory neurological disorders. Nadeau and Rivest found that acute-stress levels of GCs were both necessary and sufficient to lessen damage due to direct LPS infusion into the brain (62, 77). Thus, basal GC levels and the physiological response to acute stress are anti-inflammatory and reduce inflammatory cytotoxicity (78). In contrast, there are a number of circumstances where GCs fail to have these antiinflammatory properties. For example, dexamethasone fails to reduce inflammation in tuberculosis meningitis, although it does correspond with an improved prognosis (79) and fails to decrease cytotoxic edema following cerebral ischemia. For a recent synthesis of clinical applications where GCs do and do not have theraputic benefit see (80). Most strikingly, long-term or high stress levels of GCs appear to exacerbate similar measures of cellular inflammation. The chronic unpredictable stress (CUS) paradigm exposes animals to a different stressor each day and animals subjected to CUS after direct LPS infusion into the prefrontal cortex have markedly increased neuron and astroglia death. This effect of CUS is reversed by blocking GR, suggesting that GC signaling is the critical component of CUS involved (81). These findings are in contrast to the aforementioned protective effects of basal to low-stress levels of corticosterone on LPS toxicity. Use of the same neurological insult highlights the concentration-dependent effects of GCs on neurological inflammation. Notably, GCs also dose-dependently inhibit astrocyte proliferation (82). The pro-inflammatory GC effects extend to the extravasation and migration of circulating inflammatory cells to a site of neurological injury. This may not come as a surprise given the previously mentioned effects of GCs on these cells in the periphery. GC concentrations positively correlate with the degree of inflammatory infiltration of granulocytes and macrophages to the site 14 of hippocampal infusion of excitotoxin (63). Blocking GC synthesis reduces the migration and abundance of these cells at the injury site. Furthermore, rats exposed to chronic high stress levels of corticosterone show accelerated and augmented microglial activation and migration of peripheral cells to the injury site (63). Similarly, GCs released during CUS enhance microglial activation following direct LPS injection to the prefrontal cortex (81). These findings implicate chronic stress levels of GCs in enhanced activation of the immune cell response to neurological cytotoxicity. 1.4.4 Glucocorticoid effects on inflammatory cytokines in the CNS Levels of pro-inflammatory cytokines are an index of the inflammatory signaling cascades activated by injury. GCs potently decrease the production of these molecules in the periphery and also promote secretion of anti-inflammatory cytokines. In many cases, this is also true in the brain. Adrenalectomized or GR antagonist-treated animals have been found to have increased production of many cytokines in many different models (62, 83, 84). The studies that have looked at the effects of chronic GC exposure have also found similar results for basal GCs: locking GCs at basal levels augments excitotoxin-induced upregulation of IL-1β and TNF-α expression and protein levels in the hippocampus (63). Pharmacological inhibition of GR increases TNF-α, IL-1 beta, and iNOS protein levels in rats given LPS peripherally (85). Moreover, in hippocampal cultures, low concentrations of corticosterone inhibit excitotoxin-induced expression of IL-1β and TNF-α (86). These findings suggest that basal levels of GCs have anti-inflammatory properties on these pro-inflammatory cytokines during necrotic cell death. However, in the context of sustained stress, or stress levels of GCs, a very different picture emerges. CUS fails to suppress the increased expression of IL-1β and TNF-α following infusion of LPS into the prefrontal cortex (81). Even more striking, CUS augments TNF-α, IL-1β, and iNOS protein levels in hippocampus and cortex when LPS is administered peripherally, an effect that is GR-mediated (85). Similarly, high stress concentrations of corticosterone exacerbate excitotoxin-induced increases in IL-1β and TNF-α mRNA and protein levels both in vivo and in vitro (63, 86). A more detailed analysis of GC concentration reveals inverse-U properties where 15 intermediate concentrations enhance inflammation the most. Moderate stress levels of corticosterone are even more effective than high stress levels in augmenting excitotoxin-induced expression of TNF-α and iNOS in vitro and in vivo (86, 87). Dexamethasone causes a dose- and time-dependent increase in expression and activity of prostaglandin D2 synthase in neuronal cultures (88) and dexamethasone or corticosterone treatment increases the pro-inflammatory leukotriene synthesis enzyme 5-lipoxygenase in both the hippocampus (89) and macrophages (90). Finally, dexamethasone alone or more robustly in conjunction with retinoic acid, causes increased COX-1 expression in neuroblastoma cell lines (91). 1.4.5 Glucocorticoid effects on inflammatory transcription factors As discussed, a classic anti-inflammatory GC action in the periphery is to decrease the activity of NFκB. Commensurate with that, in unstressed rats, GR antagonists increase LPSinduced NFκB DNA binding activity in the frontal cortex and hippocampus (85). Moreover, dexamethasone treatment decreases basal NFκB DNA binding activity in cortex and hippocampus (92, 93) and also following LPS treatment (94) (however there are some caveats to this interpretation that will be discussed later.) In contrast, CUS increases LPS-induced NFκB DNA binding and protein levels in these brain structures, a GR-mediated effect (85, 87). Moreover, CUS following direct LPS infusion into the prefrontal cortex increases components of the MAPK signaling pathway involved in inflammatory pathogenesis including phosphorylated JNK, p38, and ERK (81). These effects of CUS are also GR-mediated. Taken together, these findings suggest that as for other aspects of neuroinflammation, basal GC levels decrease pro-inflammatory transcription factor signaling while high GC levels enhance it. 1.5 Overview of experimental hypotheses These findings support the hypothesis that some types of GC exposures might increase inflammation in the CNS instead of decreasing it. This suggests that the effects of GCs on the inflammatory response are actually detrimental to neurons, and might contribute to GCendangerment of neurons. The research presented in this dissertation sought to address the 16 following gaps in our knowledge about the effects of GCs during acute CNS injury: 1.5.1 Primary hypotheses tested The primary goal of this work was to determine the cellular and molecular nature of GCaugmented inflammatory responses during acute CNS injury. It might seem obvious that GCs would affect immune cells directly to increase inflammatory responses, but additional possible explanations include GC effects on neuron vulnerability or astrocyte functions which could indirectly lead to an increased immune response. Hypothesis: Exogenous GC treatment augments inflammatory responses in immune cells directly, not by increasing neuron death. In addition to the effects of exogenous GC-treatment, these studies also sought to determine the normative function of endogenous GCs on immune cells during acute neurological injury. Hypothesis: Endogenous GCs released during the stress-response also activate immune cells. This work is described in chapter 2. The existing literature reports that GCs increase a variety of different pro-inflammatory mediators like NF-kB. In addition, GCs fail to induce some of their normal anti-inflammatory targets like MKP-1, IL-1ra, and IkBa in the forebrain (87). GCs might augment inflammation via several different possible mechanisms: Hypotheses: (i) GCs increase chemokine production thereby increasing immune cell recruitment; (ii) GCs decrease anti-inflammatory signaling molecules; and, (iii) GCs increase NF-kB activation leading to increased transcription of pro-inflammatory genes. This work is described in chapter 3. The lack of insult specificity of GC neuron endangerment predicts that GCs are affecting high-level processes that are broadly deleterious to neuron survival. In agreement with this prediction, the known mechanisms for GC endangerment (impaired glucose uptake, glutamate reuptake, calcium extrusion, and ROS scavenging) could affect neuron death from a variety of different injury types. The emerging evidence that GCs can increase immune responses depending on the dose and temporal context of their exposure (7, 9) predicts that GC effects on the immune response might also contribute to GC endangerment. Hypothesis: GC-augmented 17 inflammation during acute CNS injury is detrimental to neuron survival. This work is described in chapter 4. 1.5.2 Secondary hypotheses tested Several factors contribute to the difficulty in determining GC effects. First, GCs are subject to multiple levels of regulation from the time they are released into the blood until when they act on the GC receptor (GR) so blood hormone levels do not necessarily predict GR signaling within a single cell of a complex tissue (e.g. differential binding to CBG carrier proteins in the blood, local inactivation by 11beta-HSD enzymes). Second, GR signaling is likely to carry different consequences depending on the cell type in which it occurs and there is widespread GR expression throughout many cell types in the CNS (14). Third, GC effects are sensitive to multiple factors like the timing and duration of GC exposure, whether the GCs are synthetic or the endogenous ones released post-injury, and the specific tissue in which GCs are acting. Finally, GCs affect not only the genes that they directly activate, but also those regulated by other transcription factors that GR interacts with in trans, potentially masking the original cause of the effect. In the course of investigating the primary hypotheses about GC actions, several additional hypotheses were addressed as a side-effect of developing the treatment parameters necessary to resolve the confounding issues described above. The timing and duration of GC exposure is a good example of this. Acute GC exposure prior to injury promotes increased inflammatory responses to a variety of CNS and peripheral injuries in rodents (24, 48, 95) and in humans (26, 49). This GC priming effect on the subsequent inflammatory response can be prevented by either giving GCs after injury (23, 95) or giving them chronically (for weeks) (33). Thus, at some point there is a switch from acute GCs stimulating immune responses to chronic GCs suppressing immune responses (96). To begin to assess when this switch might occur, a subacute duration of GC exposure was chosen for exogenous GC treatments in these studies. These GC treatments began three days prior to injury, so they also implicitly test the ability of this timing and duration of GC exposure to affect inflammation and injury. 18 Most of the previous observations of GC-augmented inflammation were made in rats (63, 81, 85-87) and many examples of GC endangerment are from rats as well (97). To be able to utilize the molecular genetic tools available in mice, these initial observations and parameters had to be extended to the C57Bl6/J mouse line. The application of experimental parameters from rats to mice had to be determined empirically, so an additional side-hypothesis that was tested was the ability of GCs to augment inflammation and neuron death in mice. 19 Chapter 2: GC Activation of CNS Immune Responses Because of the wide variety of GC effects on many different cell types, the unusual finding that sustained exposure to GCs can actually augment CNS inflammation could be easily dismissed as a side-effect of one of their many other possible targets. To determine whether GCs were activating the immune response directly via their actions on immune cells themselves, two separate approaches were employed simultaneously. The first approach was to develop a mouse genetic model where GR could be deleted or overexpressed in specific cell types, and the second approach was to manipulate hormone levels and use pharmacological antagonists to study the same effects in rats. These two approaches were largely synergistic and are considered together throughout the rest of this dissertation. 2.1 Experimental model To determine the role of stress-level GCs in neurons and myeloid cells during acute neurological injury, homozygous floxed-GR mice (98) were crossed to either LysM-CRE (myeloid GR-KO, MGRKO) or CamKIIa-CRE (forebrain GR-KO, FBGRKO) driver mice. These conditional KO mice have GR deleted throughout myeloid-lineage cells (MGRKO) (99) or forebrain neurons (FBGRKO) (100) and were an extremely generous gift from Dr. Louis Muglia (Vanderbilt University). 2.1.1 MGRKO mice have GR deleted in myeloid cells Although MGRKO mice are described to have efficient myeloid-cell lineage deletion of GR (99), we wanted to determine the efficiency of this recombination in CNS myeloid cells. To quantify the percentage of myeloid cells lacking GR, these cells were isolated from WT and MGRKO brains on a percoll gradient (101). The extracted mononuclear cells were pooled from 5 animals and GR levels were quantified by western blot. This analysis found that MGRKO mice have 33.6% (±11.1% s.e.m.) reduction in total GR protein levels compared with their WT littermates (Fig. 1a). The extraction of myeloid cells from the whole brain includes many different subtypes of these cells including perivascular macrophages and parenchymal microglia. To determine whether 20 parenchymal microglia in the regions we were interested in have efficient GR recombination in MGRKO mice, tissue sections were stained with an antibody for the GR and a myeloid cell marker. We used CD68 because it is expressed at low levels in inactive monocytes in cytoplasmic granules and inactive GR would also be expected to be present in the cytoplasm. Many but not all CD68+ cells were missing the GR in the MGRKO cortex (Fig. 1b) and in the MGRKO hippocampus (Fig. 1c). Because only exon 1C of the GR is floxed in these mice, it is possible that C-terminal fragments of the GR are still produced. We found a similar lack of GR immunoreactivity in MGRKO brains stained with antibodies raised against the N-terminal or Cterminal end of the GR, arguing against this possibility (Fig. 1c). Other investigations using the LysM-CRE driver have reported that its expression is largely specific to myeloid cells, (99, 102) and we verified this for the brain regions we intended to study in two additional ways. First, LysM-CRE mice were crossed to a ROSA-lacZ reporter mouse that expresses the beta-galactosidase gene in cells undergoing CRE recombination. These mice express this reporter throughout the cortex (Fig. 2a) and hippocampus (Fig. 2c), and antibeta-galactosidase staining co-localized with CD68+ cells (Fig. 2d). Finally, staining for the CRE enzyme itself revealed a similar co-localization with myeloid cells (Fig. 2b). 2.1.2 FBGRKO mice have GR deleted in forebrain neurons FBGRKO mice have efficient deletion of the GR in forebrain neurons after 4 months of age (100). We tested the expression pattern of the CamKIIa-CRE promoter using the ROSA-lacZ reporter and found widespread expression throughout forebrain neurons as early as 3 months of age (Fig. 3a). Expression in the cortex and CA3 region of the hippocampus particularly, was further enhanced by allowing animals to be 5 to 6 months old. To determine whether this CRE driver could detectably reduce GR protein levels in the whole brain, infarcted hemispheres were collected 24 h post-MCAO and analyzed by western blot with the N- or C-terminal anti-GR antibodies (Fig. 3b). Although neurons were not separated from other cells, a decrease in GR immunoreactivity of 33.6% (± 32.9, 95% CI, N-terminal antibody) or 15% (± 8.6, 95% CI, Cterminal antibody) was still detectable. We further verified this deletion by staining for the GR in 21 WT and FBGRKO littermates and found areas of missing GR expression in the pyramidal CA3 neurons in the hippocampus, even though these animals were only 2 months of age (Fig. 3c). 2.1.3 MGROV and FBGROV mice have inducible overexpression of GR in myeloid cells or neurons In addition to testing the need for GR signaling for various effects during acute CNS injury, we also wondered whether some of those same effects could be enhanced by increasing GR protein levels. To accomplish this, the same CRE-drivers were crossed to mice harboring ROSA::loxP-stop-loxP-rtTA (from Jackson Labs) and CMV-TRE-rGR that was generously provided by Dr. Sam Okret. These mice overexpress GR in myeloid cells or neurons upon administration of doxycycline (DOX) (Fig. 4a). To verify overexpression, MGROV and WT mice were given DOX in their drinking water for 7 days and monocytes were isolated on a percoll gradient. Anti-GR immunoblotting determined that GR overexpression was 1.76 times greater (±0.39, 95%CI) in microglia isolated from MGROV brains than in microglia from WT brains (Fig. 4b). In neurons, GR overexpression was clearly higher in forebrain neurons of mice that had been given DOX for longer than 3 days (Fig. 4c,d). These observations suggested an experimental design where GC manipulations begin on day 5 of DOX to take advantage of the increased GR protein levels and fresh DOX is given regularly including 24 h prior to any significant manipulation (Fig. 4e). One caveat to consider with this method of overexpressing the GR is its reliance on DOX to induce the overexpression. DOX has neuroprotective and anti-inflammatory properties, so all experiments were done with WT and overexpression mice receiving the same DOX treatments. Despite this, infarct volumes were smaller in DOX-treated mice and DOX could interfere in unknown ways depending on the treatment (e.g. if GC-treatment affected inflammatory parameters that were also more affected by DOX). 2.1.4 GC levels in experimental system Having verified that MGRKO and FBGRKO mice have efficient and specific deletion of the GR in the expected cell types, we next wanted to determine how the various GC 22 manipulations affected GC levels. It is possible that MGRKO mice have an abnormal stress response so to test this we exposed them to 2 hours of immobilization stress and measured serum corticosterone levels (Fig. 5a). Immediately post-immobilization, MGRKO mice had slightly lower cort levels, but by 2 h post-stress, GC levels were back to normal in both groups. GC levels at 6 h were around 10 µg/dL, consistent with a normal rise in circadian release, and there were no lasting effects by 24 h. These data suggest that the stress response in MGRKO mice is largely intact. In contrast, FBGRKO mice have elevated GC levels (100), as would be expected from deleting the negative feedback from GR signaling in hippocampal neurons (1). Comparison of WT and KO mice was used to assess the role of the endogenous GC signaling in each cell type. To measure the role of exogenously administered GCs, animals were implanted with a 10 mg s.c. corticosterone pellet. Corticosterone (CORT) was chosen instead of pharmacological GCs because the latter are targets of the mdr-1 protein in the brain and could actually lead to a decrease in corticosterone centrally, as less endogenous GCs are produced in the presence of synthetic GCs (15). To determine the blood CORT levels achieved following pellet implantation, weight-matched WT mice were given pellets and serum CORT levels were measured 1, 3, or 6 days later (Fig. 5b). CORT levels exhibited a linear decrease over time, with a pooled slope of -10.96 µg/dL/day, and a pooled Y-intercept of 102.0 µg/dL. A separate cohort of WT mice was measured post-KA to see if blood CORT levels were still elevated 24 h after injury (4 days post-pellet) or 72 h later (6 days post-pellet). Pellet-implanted animals still had serum CORT levels equivalent to if they had not received KA and were still substantially elevated over no pellet controls after 4 or 6 days (Fig. 5b). Finally, KA treatment on day 3 did not significantly alter the slope of the line Together, these results defined the exposure to exogenous GCs that was achieved by s.c. pellet implantation and helped to suggest the timing of the experimental design in Fig 4e. 2.1.5 Injury models After establishing the parameters of the GC treatments, mice were next subjected to two different acute neurological injuries. The two injuries selected were the excitotoxin KA and the 23 distal model of MCAO. These injuries affect the hippocampus and the cortex, respectively, brain regions with high GR abundance (15), and where GCs have been found to increase inflammation and neuron death (63, 81, 85, 87). Furthermore, they share some but not all of the same pathological characteristics so their comparison might indicate how generalizable the effects of GCs are across injury models. Many of the original studies that observed GC-augmented inflammation and neuron death were performed in rats, and the ability to observe these same responses in mice was not a guarantee. Thus, several experiments in rats were conducted in tandem with the mouse experiments. This parallel set of investigations used the same KA model of excitotoxicity that was used in the mice where KA was injected stereotactically into the hippocampus. This provided a unique opportunity to investigate similar questions about how GCs affect CNS injury and inflammation in separate model organisms using very different experimental approaches. In rats, the manipulations were gross physiological changes or pharmacological interventions, whereas in mice the manipulations were largely genetic. The fact that these very different investigation styles yielded similar conclusions was nothing short of remarkable, and throughout the rest of this dissertation the findings from rats and mice are presented together, organized by the inflammatory parameters that were measured. The experimental design timeline for the rat studies is shown in Fig. 15a, and a similar timeline for the mouse experiments is shown in Fig. 16a. Mouse overexpression experiments utilized the slightly modified experimental design indicated in Fig. 4e. 2.2 Results 2.2.1 GR signaling in myeloid cells increases immune cell activation and recruitment To measure immune cell activation in KA-treated mice, sections were stained for CD68, a myeloid cell marker that is upregulated in activated cells (Fig. 6a). In WT mice, exogenous GCtreatment increased CD68+ area as well as the pixel intensity of that signal 72 h after KA (Fig. 6b,c). MGRKO mice still had the same GC-mediated increase in CD68+ area but had a reduced pixel intensity of the CD68+ signal within that area (Fig. 6c). 24 To measure immune cell activation in mice post-MCAO, myeloid cells were isolated from the infarcted hemisphere on a percoll gradient and labeled with CD11b+, CD45, and Ly6 antibodies for analysis by flow cytometry (Fig. 7a). Cells that were CD45+ and CD11b+ (Fig. 7b) were gated based on their forward and side scatter profile and split into three populations (Fig. 6d–f). Granulocytes are Ly6G+CD45low (P1) whereas activated mononuclear cells are Ly6GCD45high (P2). The largest population, however, was the resting microglia Ly6G-CD45low (P3) outside of the injury site that were extracted along with the activated cells. These populations were quantified as a percentage of the parent gate (G2) and to control for variability between extractions, the fold increase in each population % was expressed relative to that population’s % in the contralateral hemisphere (representative dot-plots are shown in Fig. 7c). This yielded an estimate of the change in each population due to the stroke infarct, or an approximation of the recruitment of these cells into the injury site. GCs affected the recruitment of granulocytes and monocytes similarly, but exogenous and endogenous GCs had divergent effects on immune cell recruitment. Treatment with exogenous GCs suppressed monocyte recruitment (Fig. 6h) and there was a trend towards the same effect for granulocytes (Fig. 6g). This is the standard immunosuppressive effect that longer exposure to GCs can have on immune cell recruitment, and it required GR signaling in monocytes to occur because GC-treatment did not suppress granulocyte or monocyte recruitment in MGRKO mice. In contrast, GR signaling in myeloid cells was actually required for normal levels of granulocyte recruitment (Fig. 6g) and there was a trend towards the same effect for monocytes (Fig. 6h). Thus, while GC-treatments suppressed peripheral inflammatory cell recruitment via GR signaling, the recruitment of these cells also required normal GR signaling to occur. This analysis did not detect any differences in the resting microglial cell populations (Fig. 6i). To see if GR is also required in immune cells for their recruitment outside of the CNS, mice were tested in a model of peripheral inflammation. Leukocytes were collected in Zymosan A after a week of inflammation induced by s.c. injections of sterile air. More than a 50% reduction in leukocyte recruitment was detected in MGRKO mice (Fig. 6j), suggesting that GR 25 signaling in immune cells is required for their migration to many injury sites not just those in the CNS. Finally, because GR signaling was necessary for both stimulation and suppression of immune cell recruitment, the effects of GR overexpression on these populations were measured. GR overexpression alone did not significantly alter any of the cell populations (Fig. 7d–f). Thus, GR signaling in myeloid cells is necessary but probably not sufficient to mediate GC-augmented inflammatory responses to acute CNS injury. 2.2.2 GCs increase immune cell activation in the injured rat hippocampus Sub-acute (72 h) GC exposure prior to injury endangers neurons and increase inflammation following KA-induced excitotoxicity in rats (63). This injury model was therefore selected to study GC-augmented inflammation and its relationship with neuron death in rats. Previous examples of GC-augmented inflammation compared intact rats with adrenalectomized (ADX) rats, where the endogenous source of GCs is removed. We wanted to ensure that GCaugmented inflammation was not simply an artifact caused by ADX so in this study we compared ADX rats given either low or high GC replacements to isolate GC-dose as a variable. These treatments lock blood GCs at either basal (low-GC group, 2-4 µg/dL) or stress-levels (high-GC group, 30 µg/dL (87)). Three days later during kainic acid-induced excitotoxic injury, rats were also given injections of either indomethacin or minocycline (Fig. 8a). Three days post-injury, brain sections were stained for activated infiltrating and resident immune cells (CD11b/c) and astrocytes (GFAP) in the injury site (Fig. 8b-e). Intact rats were also evaluated as a reference; however, the primary comparison was between low-GC and high-GC rats where GC exposure was a constant, controlled variable. In support of GCs augmenting cellular inflammation, GCs doubled the CD11b/c signal (Fig. 8b,c). This increase could be blocked by concurrent treatment with minocycline, an inhibitor of microglia activation. Minocycline also reduced GFAP signal in astrocytes by approximately one-half (Fig. 8d,e). In contrast, the non-selective COX-inhibitor indomethacin had no effects on CD11b/c or GFAP signal. These results confirm that GCs are sufficient to increase cellular 26 inflammatory responses and show that GC-augmented inflammation can be suppressed by minocycline but not indomethacin treatment. 2.3 Discussion 2.3.1 Endogenous and exogenous GCs affect different aspects of cellular inflammation postinjury In agreement with these observations in KA-treated rats and similar published findings in rats (63), GC-treatment increased CD68 area by 1.5 times and CD68 intensity by 1.75 times in WT mice. This observation supported the idea that GC-augmented inflammation is a general enough phenomenon that it could be observed in multiple rodent models. Removing GR in myeloid cells did not suppress the GC-mediated increase in CD68 area, but it did reverse the pixel intensity of that signal. All treatment groups were counterbalanced during the IHC staining protocol supporting pixel intensity as a rough estimate of CD68 levels and CD68 area as a rough estimate of the size of the microglia population. This suggests that GR signaling is required in these cells for GCs to activate immune cells, but not for cell proliferation in response to injury. Following MCAO, exogenous GC-treatment suppressed peripheral myeloid cell recruitment and had a trend towards the same for granulocytes (Fig. 6g,h). This is in agreement with literature demonstrating similar suppressive effects of exogenous GC treatments on leukocyte recruitment to other injuries (18, 24). Furthermore, this GC-mediated suppression of recruitment required GR in the immune cells to occur as the LysM-CRE driver is heavily expressed in granulocytes and monocytes alike. Although myeloid-cell GR signaling was necessary for GCs to suppress their recruitment, it was also necessary for their recruitment in the first place. MGRKO mice had a significantly lower number of granulocytes and a trend towards fewer monocytes (Fig. 6g,h), suggesting that stress-activation of GR in myeloid cells normally is required for their recruitment to the injury site. In support of this not being an effect limited to the CNS, intact GR signaling in myeloid cells was also required for proper leukocyte recruitment to a peripheral site of inflammation (Fig. 6j). 27 These results suggest that stress-mediated GR signaling in myeloid cells is required at a proper level or during a proper time for those cells to migrate to an inflammatory site. Thus, endogenous GR signaling is required in myeloid cells for proper cell activation and recruitment to the injury site. Exogenous GCs increased cellular activation in the KA model and suppressed peripheral cell recruitment in the MCAO model. 2.3.2 GCs increase immune cell activation in the injured hippocampus To see whether the amount of inflammatory cell activation post-injury could be manipulated pharmacologically, rats were treated with two anti-inflammatory drugs with alternative mechanisms of action, indomethacin and minocycline. Indomethacin is a non-selective inhibitor of the COX enzymes that are the rate-limiting step in the production of prostaglandins. Two months of treatment with indomethacin only slightly reduces microglial activation postirradiation (103) so we did not expect this drug to have a large effect in this injury model. In contrast, minocycline is reported to be a potent inhibitor of microglial activation (104). This fits with the finding that CD11b/c+ area was slightly (but not significantly) reduced by indomethacin and was robustly suppressed by minocycline (Fig. 8b,c). Comparison of low-GC and high-GC rats revealed that higher GC doses increase CD11b/ c+ area (Fig. 8b,c). This agrees with previous reports that GCs increase immune cell activation both basally (105) and in this same excitotoxicity model (63). Previous work using this same excitotoxicity model found that ADX reduced inflammatory cell activation and intact rats given additional GCs had even greater inflammatory cell activation (63). These effects could potentially be due to compensatory decreases in endogenous GC synthesis (for the comparison of intact with intact given additional GCs) or they could be an artifact of ADX (for the comparison of intact with ADX). The present finding that GC-augmented inflammation still occurs when GC dose is an isolated variable in ADX rats argues against these alternative possibilities. For these ADX studies, only certain comparisons could be used to reach these conclusions. Intact rats release endogenous GCs in response to the stress of receiving KA-injury, and also retain their normal circadian rhythm in GC release compared with ADX rats. Although 28 ADX rats were given physiologically relevant stress-GC levels (106), implanted GC pellets cannot mimic the pulsatile release of endogenous GCs (107), and ADX also removes catecholamines. Comparison between ADX rats was used to draw conclusions about the effects of constant stress-level GCs relative to constant basal GCs. Comparison of either of these groups to intact rats is included as a useful reference, but there are several uncontrolled variables between these groups so care must be taken when interpreting the underlying cause of any differences. Thus, the primary conclusions drawn from this study are based on comparison of low-GC and high-GC rats where GC exposure was a constant, controlled variable. 2.4 Conclusions The goal of these investigations was to measure how endogenously released GCs and exogenous GC treatments affect the immune response to acute CNS injury. Despite being exposure to the same GC, corticosterone, endogenous and exogenous GCs could differ in their effects due to differences in the timing and duration of exposure. Endogenously-released GCs were pro-inflammatory in that they increased CD11b/c+ signal in rats post-KA and they increased granulocyte and, likely, monocyte recruitment in mice post-MCAO. Exogenous GC-treatment also had pro-inflammatory effects including increasing CD11b/+c signal in rats post-KA and CD68+ signal in mice post-KA. GCs augmented the area and the intensity of the CD68+ signal but only the increase in intensity of the signal required GR signaling in myeloid cells to occur. These effects strongly support both of the original hypotheses that exogenous GC treatments augment inflammatory responses in immune cells directly, and that endogenous GCs released during the stress-response also activate immune cells. Finally, these results suggest an explanation for the immunosuppressive effects that are often reported for exogenous GCs: such treatment strongly reduced the recruitment of peripheral monocytes and likely granulocytes to the infarct post-MCAO and this effect of exogenous GCs was myeloid-cell GR-dependent. Because the GC-augmented cellular activation required GR signaling in myeloid cells, it suggested the possibility that GCs in fact were activating myeloid cells directly, continuing to challenge the established dogma that sustained stress level GC exposure is immunosuppressive. 29 In an effort to further understand the basis for these effects, the effects of GCs on a number of inflammatory mediators were measured. The next chapter explores some of the potential molecular explanations for how GCs are increasing cellular inflammatory responses to acute CNS injury. 30 Chapter 3: Mechanisms of GC-Augmented Immune Responses 3.1 Results Next, several non-competing hypotheses about the molecular mechanisms behind this GC-augmented cellular inflammation were tested: (i) GCs increase pro-inflammatory cytokine and chemokine production thereby increasing immune cell activation; (ii) GCs decrease antiinflammatory signaling molecules; and, (iii) GCs increase NF-kB activation. Any or all of these could be explanations for the increased cellular inflammatory response described in chapter 1, as they all reflect GC actions that are counter to their normal anti-inflammatory effects. Even a failure to suppress pro-inflammatory cytokines or a failure to induce anti-inflammatory cytokines could potentially explain GC-augmented cellular responses since GCs normally play a role in the recovery from the initial inflammatory response and GC-augmented inflammation could simply be a failure of this recovery to occur. 3.1.1 Hypothesis (i): GCs increase pro-inflammatory cytokines and chemokines One possible explanation for GC-enhanced immune activation is that GCs increase the signals that activate or recruit immune cells. The injured CA3 region was micro-dissected at several time points post-injury to measure the levels of two principal chemokines involved in recruiting monocytes (CCL2) and granulocytes (CINC-1) (Fig. 9). GCs did not significantly alter the release of either chemokine, suggesting that GC-augmented inflammation occurs via another mechanism. ADX did affect the release of these chemokines, however, slightly hastening their production regardless of GC treatment. This re-emphasizes the need to control for ADX as a confounding factor when attempting to study the effects of GCs. Previous reports of GC-augmented inflammation detected increased production of the pro-inflammatory cytokine IL-1beta in response to an inflammatory challenge (63, 81, 85-87) and this might explain an increased cellular response. Prior to injury, both low and high GC rats had 2-3 times more IL-1beta than intact rats, but high GCs did not increase IL-1beta levels relative to low GCs (Fig. 10c). This suggested that while they may not stimulate more IL-1beta production basally, GCs are at least partially deficient in their ability to suppress IL-1beta production. To see 31 if this deficiency involves GR signaling in myeloid cells, IL-1beta and IL-6 levels were measured in the different KO mice. IL-1beta and IL-6 levels were measured in MGRKO and FBGRKO mice and their WT littermates at 12 h post-KA and 24 h post-MCAO to determine the cell-specific role of GR signaling in their production (Fig. 12a–d). Following KA, exogenous GC treatments slightly, but not significantly, increased IL-1beta and IL-6 levels and this effect was significantly enhanced in MGRKO but not FBGRKO mice (Fig. 12a,c). Following MCAO, exogenous GC treatments suppressed IL-6 and had a trend towards the same effect on IL-1beta. In MGRKO mice, this exogenous GC suppression of IL-6 did not occur and the trend for IL-1beta was reversed (although still not significant) (Fig. 12b,d). FBGRKO mice had only minor differences in their cytokine response, irrespective of treatment or injury type. Finally, the effects of GCs on iNOS and COX-2 were measured because these enzymes are rate limiting in the synthesis of NO and prostaglandins in response to injury. T-cell GR is required for a lethal activation of COX-2 during peripheral inflammation (98). During stroke, the production of NO and prostaglandins is particularly detrimental and GCs can activate either of these systems with potentially deleterious consequences for neurons (22, 108-110). GR signaling was not required for normal iNOS induction post-stroke (Fig. 12e). In contrast, COX-2 induction was reduced in FBGRKO but not MGRKO mice relative to WT controls (Fig. 12f). These results demonstrated that GR signaling in neurons is necessary for COX-2 induction post-MCAO. These findings indicated that GCs are unlikely to increase cellular inflammatory responses by increasing the production of pro-inflammatory signaling molecules. In some cases, GCs suppressed these molecules, but in more situations GCs had little effect. 3.1.2 Hypothesis (ii): GCs decrease anti-inflammatory signaling molecules An alternative explanation for GC-enhanced immune activation is that GCs decrease antiinflammatory signals prior to injury. In the CNS, reduced expression of CX3CL1, CD200, and CD22 is associated with increased immune cell responses (111-114). To determine whether the GC treatments affected the expression of these genes, their levels were measured by qPCR 32 immediately prior to KA-injury (Fig. 10a). The expression of TGF-b1 was also measured because GCs normally induce this gene as a component of their anti-inflammatory actions (1, 14). In agreement with the cellular activation profile post-injury (Fig. 8b,c), GCs significantly reduced the expression of several key anti-inflammatory signaling molecules in the uninjured rat hippocampus. Relative to high-GC rats, low-GC rats had 2 to 4-fold increases in the expression of CX3CL1, its receptor CX3CR1, and CD22 (Fig. 10b). Furthermore, GCs failed to induce TGFbeta or IL-1ra, targets that GCs activate as a component of their anti-inflammatory actions and the recovery from the inflammatory response. Thus, GCs suppressed several anti-inflammatory signaling molecules and failed to induce some of their known anti-inflammatory targets in the hippocampus. GC-mediated reductions in CX3CL1 were of particular interest because of the magnitude of the effect and the possibility that such reductions could increase the toxicity of the inflammatory response to neurons (115). To determine the cell-specificity of the effects of GCs on CX3CL1, and also to measure GC effects on this chemokine post-injury, western blots for CX3CL1 were quantified at 12 and 72 h post-KA or at 24 h post-MCAO in WT and MGRKO and FBGRKO mice (Fig. 11). By 12 h post-KA, GC treatments in intact WT mice had the same effect in reducing CX3CL1 as GC treatments in ADX rats. This effect could be blocked by knocking out the GR in either neurons or myeloid cells. After 72 h, GR signaling in myeloid cells but not neurons was required to maintain reduced CX3CL1 levels (Fig. 11b,e). These results demonstrate that GR signaling in myeloid cells maintains reduced CX3CL1 levels in the hippocampus and that GCs require intact GR signaling in both neurons and myeloid cells to suppress CX3CL1. In contrast, at 24 h post-MCAO, CX3CL1 levels were highly increased by GC treatment (Fig 11c,f). These increases occurred to nearly the same degree in WT and KO mice, regardless of whether GR was deleted in neurons or myeloid cells. Furthermore, GR signaling in both neurons and myeloid cells was necessary for increased CX3CL1 levels. Thus, GCs stimulate CX3CL1 levels in the cortex post-ischemia, but they suppress and maintain a reduced CX3CL1 level in the hippocampus post-KA. 33 3.1.3 Hypothesis (iii) GCs increase NF-kB activity If GCs decrease anti-inflammatory signals and increase cellular activation, then they might be expected to do so by altering the transcriptional activity of one of their primary targets, NF-kB. We measured the nuclear activity of the p65 subunit of NF-kB via EMSA immediately prior to KA-injury (Fig. 10d) but there were no differences in baseline NF-kB activity following GC-treatment. Thus, the expression changes in anti-inflammatory cytokines are not likely to be mediated via changes in NF-kB DNA binding activity. GCs mediate their classical anti-inflammatory effects on NF-kB activity either directly by protein–protein interaction or indirectly by inducing the inhibitor of NF-kB, IkBa (14). The inability of GCs to alter baseline NF-kB activity in the hippocampus prompted us to investigate whether our GC treatments induced IkB in this brain region. Indeed, they did not (Fig. 10e), and as a control we assayed IkB expression in the frontal cortex, another brain region with abundant GR expression and found normal IkB induction by GCs there (Fig. 13c,d). This suggested that GCs might have an impaired ability to inhibit NF-kB in the hippocampus. To see if GCs could affect NF-kB activation post-injury we measured its activity at the peak of the immune response (24 h post-KA in Fig. 9). High-GCs suppressed NF-kB activation by 25.4% (±22.8%, 95%CI) relative to low-GCs (Fig. 13,b), demonstrating that GCs can at least modestly reduce NF-kB activation in the hippocampus. In contrast, however, several different intermediate GC levels (corresponding to moderate stress) increasingly activated NF-kB. This suggested that NF-kB activation depends on GC dose, and given that NF-kB activation also has different consequences depending on the cell it is activated in, we next measured the effects of the cell-specific GR manipulations on NF-kB activation. Endogenous and exogenous GCs increased p65 levels after both injuries in mice. Endogenous GR signaling is necessary for normal p65 activation in WT animals regardless of whether it happens in myeloid cells (Fig. 14b,c) or neurons (Fig. 14e,f) at any time point following either injury. Exogenous GC treatment also potentiated p65 activity in both myeloid cells and neurons individually, and removing GR in either cell type was insufficient to completely 34 reverse the effect. By 72 h post-KA, or 24 h post-MCAO, however, the MGRKO mice had significantly suppressed p65 levels, suggesting that GR in these cells is necessary for more prolonged p65 activation. These results are consistent with both endogenous and exogenous GCs stimulating p65 activation in myeloid cells and neurons independently. 3.2 Discussion 3.2.1 Hypothesis (i): GCs do not increase pro-inflammatory cytokines and chemokines Given that GCs doubled immune cell activation, a likely possibility is that they do so by accelerating and/or increasing the magnitude of chemokine release in the injury site. The predominant chemokine source is inflammatory cells themselves, so increased cellular activation in the presence of GCs might correspond with higher chemokine levels. Instead, we found that CCL2 and CINC-1 levels post-injury were unchanged by GC-treatments (Fig. 9) suggesting either that GCs are suppressing chemokine production, or that greater cellular activation does not equate with more chemokine production. From this we concluded that GCs must affect other mechanisms to increase immune activation. In other examples of GC-augmented inflammatory responses, GCs increase the proinflammatory cytokine Il-1beta in the uninjured hippocampus (63) and after generalized inflammation from peripheral or centrally injected LPS (81, 85, 87), or excitotoxicity (63, 86). If GCs increase the production of this cytokine prior to the injury, it might lead to augmented and potentially more neurotoxic immune responses, particularly in the context of reduced antiinflammatory signaling by molecules like CX3CL1 (115). In this study both ADX groups had 2-3 times the IL-1beta protein levels in the uninjured hippocampus, irrespective of GC dose. This finding is in agreement with other observations that ADX can increase pro-inflammatory signaling molecules in the brain (1, 116); however, high GCs should suppress IL-1beta relative to low if GCs were fulfilling their anti-inflammatory potential. IL-1beta and IL-6 were increased by exogenous GC treatments in MGRKO but not FBGRKO mice (Fig. 12a,c). This result suggests that GR signaling in myeloid cells normally suppresses cytokine levels post-KA, a commonly observed anti-inflammatory GC effect. 35 Interestingly, GR signaling only was necessary for this reduced cytokine response in the presence of additional GC treatment. This means that during normal GC-augmented inflammation, GR signaling in myeloid cells is still suppressing some of the increased inflammatory response. Following MCAO, exogenous GC treatments suppressed IL-6 and had a trend towards the same effect on IL-1beta, another typically reported anti-inflammatory effect. This exogenous GC suppression of IL-6 and possibly IL-1beta as well, required GR signaling in myeloid cells to occur (Fig. 12b,d). Thus, GR signaling in myeloid cells is required for cytokine suppression in both injury models, a classic anti-inflammatory GC effect. Finally, GCs were considerably less able to suppress these pro-inflammatory cytokines during KA injury than during MCAO. This difference in effect could be due to the considerably larger immune cell population involved in the response to stroke compared with excitotoxicity. Although GR signaling in neurons was necessary for increased COX-2 levels, it is unlikely that this effect could explain GC-augmented inflammatory responses because GCs could still increase cell activation in the presence of indomethacin, a COX-2 inhibitor (Fig. 8c). The lack of effect in FBGRKO mice indicated that GR signaling in neurons does not affect IL-1beta or IL-6 production in these injuries. This result is not surprising given that it would have otherwise had to be due to an indirect effect of neuron GR signaling on cytokines that originate in myeloid cells. Thus, these investigations found little evidence to support the hypothesis that GCs augment inflammation by increasing the production of pro-inflammatory signals. GCs did not increase pro-inflammatory chemokine and cytokine production, nor did they augment inflammatory responses via altering the levels of rate-limiting enzymes in the synthesis of these molecules. During MCAO, GCs were able to suppress IL-6 and probably IL-1beta in a manner that required GR signaling in myeloid cells to occur. During KA, GCs were significantly less suppressive of the production of these pro-inflammatory cytokines, despite the fact that GR signaling in myeloid cells was still required for that suppression, but this level of analysis did not reveal any clear explanations for how GCs are increasing inflammatory responses described in 36 chapter 1. In general, these investigations found support for traditional anti-inflammatory GC effects with a few notable exceptions where GCs simply failed to have this expected action. 3.2.2 Hypothesis (ii): GCs decrease anti-inflammatory signaling molecules In contrast, GCs were found to suppress expression and protein levels of several antiinflammatory cytokines and chemokines (Fig. 10b, 11). The anti-inflammatory genes that GCs suppress are at least partially redundant in their ability to restrain the immune response to injury. CX3CR1 is a receptor present on immune cells and its ligand CX3CL1 is a soluble factor expressed in neurons throughout the CNS (117). CX3CR1 knockout mice have a more neurotoxic microglial response to a number of different CNS injuries (115). CD22 is less-extensively studied, but is a soluble ligand for the pan-leukocyte marker CD45 and it inhibits microglial release of cytokines in response to LPS (113). That GCs affect these molecules in the CNS is not unprecedented as they have been seen to reduce CX3CL1 expression in respiratory epithelial cells (118). Thus, exposure to GCs reduces the expression of several critical inhibitory inflammatory signals in the hippocampus. Following an injury, GCs suppress CX3CL1 by displacing NF-kB from the CX3CL1 promoter (118), but in the absence of a stimulus NF-KB is not present on this promoter, suggesting that in the absence of injury, GCs must suppress CX3CL1 via an alternative method and that GC suppression of CX3CL1 expression might be even greater post-injury. Indeed, GCs did suppress CX3CL1 levels in the hippocampus at 12 h post-KA and GR signaling in myeloid cells was necessary to maintain this reduced CX3CL1 expression at 72 h post-KA. Furthermore, these results demonstrated that GC treatments can suppress CX3CL1 in intact mice just as they do in ADX rats. These actions could lead to an increased cellular inflammatory response to hippocampal injury. The GC-augmented CX3CL1 levels in the ischemic cortex are also likely to contribute to increased immune cell activation since this chemokine is thought to mediate leukocyte chemotaxis to the injury site (119). Given the minimal effects of GCs on CCL1 and CINC-1 (Fig. 9), CX3CL1 is an attractive alternative chemokine explanation for the ability of endogenous GCs to stimulate leukocyte recruitment post-MCAO. Despite these opposing effects of GCs on CX3CL1 post-KA and post-MCAO, both alterations 37 could potentially harm neurons because CX3CL1 deficient mice had smaller infarct volumes post-MCAO (120) but CX3CR1 deficient mice exhibited more neurotoxic inflammatory responses (115). The observation that GCs have a reduced ability to suppress IL-1beta following KA in multiple studies including the present findings, suggested that in addition to suppressing antiinflammatory signals, GCs might also have a deficit in their ability to inhibit pro-inflammatory signals. Supporting this, GCs failed to suppress IL-1beta in rats, and they also failed to induce IL-1ra, the anti-inflammatory antagonist of IL-1beta (Fig. 10b) and a normal target of their antiinflammatory actions (87). To further investigate this possibility, the third hypothesis addressed the transcriptional activation of NF-kB, one of the primary targets that GCs inhibit as a component of their anti-inflammatory actions. 3.2.3 Hypothesis (iii) GCs increase NF-kB activity Aside from their suppression of several anti-inflammatory genes, the evidence that GCs also fail to induce some of their normal anti-inflammatory targets suggested that GCs might be impaired in other aspects of their normal anti-inflammatory mechanisms like NF-kB inhibition. GC treatments did not affect basal NF-kB activation (Fig. 10d) but did affect post-injury NF-kB DNA-binding (Fig. 13a,b), and primarily activated NF-kB in the hippocampus, except for a concentration-dependent inhibition that occurred in animals with blood GC levels higher than 30 µg/dL (high GCs), (Fig. 13b). Despite this dose-dependent inhibition, high GCs did not reduce NF-kB activation below the level of intact rats, an anti-inflammatory effect that would be expected from such constant high-dose exposure. This matches with other observations following peripheral LPS where high GCs did not suppress NF-kB activity below intact levels in the hippocampus, but were more than two-times more suppressive of NF-kB in the frontal cortex (87). Correspondingly, GCs could induce IkBa better in the frontal cortex than in the hippocampus (Fig. 13c,d), suggesting that IkBa induction might be necessary for effective suppression of NF-kB by GCs in the hippocampus. GC induction of IkBa is specific to certain 38 cell types (14), so this difference could extend to brain regions populated by distinct neurons and glia. In the rat KA model, intermediate GCs were the most stimulatory of NF-kB activity (Fig. 13a,b), demonstrating an inverse-U response where moderate stress has the largest proinflammatory effects. This matches with similar augmented inflammatory responses observed during chronic mild stress (81, 85) or daily GC injections, both of which would frequently expose animals to intermediate concentrations (63). An identical dose-response analysis of GCtreatments following peripheral LPS identified the same enhancement of LPS-induced NF-kB activity by intermediate GC concentrations (87). Activation of NF-kB leads to an increased inflammatory response and inhibition of this is a primary means by which GCs are anti-inflammatory so the finding that GC-treated mice have increased p65 nuclear localization is in opposition to this. The CD68 cell activation corresponds with p65 levels and both are reduced in MGRKO mice at 72 h post-KA suggesting that GR signaling in myeloid cells is necessary for this increased cellular activation in terms of both markers. Unexpectedly, MGRKO mice were not protected from p65 potentiation at 12 h post-KA, but by 72 h post-KA or 24 h post-MCAO, GC-treatment could no longer increase p65 levels in the absence of myeloid cell GR signaling (Fig. 14a–c). These time-dependent effects of myeloid GR signaling could reflect separate p65-augmenting effects of GCs in neurons (perhaps induced by the initial inflammatory cascade from acute injury) and p65-augmenting effects of GCs on immune cells after many have been recruited. Finally, at any time point in either injury model, removing GR in either myeloid cells or neurons decreased nuclear p65 levels. This suggests that endogenous GCs are necessary for increased p65 nuclear translocation and that they do this in both neurons and immune cells. Taken together, these findings suggest that endogenous GCs are necessary for normal p65 activation in neurons and in myeloid cells independently. In contrast, exogenous GCs activate p65 to a greater extent and maintain its elevation via signaling predominantly in myeloid cells themselves. This prolonged activation is very likely to contribute to damage in the injury site, and indeed elevated 39 NF-kB signaling in myeloid cells is required for neuron death in both of these same injury models (102). 3.3 Conclusions The pro-inflammatory effects of GCs began as an assortment of observations about increased and decreased inflammatory mediators during a number of different immune challenges and model systems. Chapter 2 characterized this augmented response during two different acute neurological injuries and asked which effects could be attributed to GR signaling in myeloid cells. To further understand the molecular mechanisms behind these increased inflammatory responses, this chapter systematically measured the effects of various GC treatments on pro- and anti-inflammatory cytokines as well as other well-known mediators of inflammation. These results identify several anti-inflammatory signaling molecules that are suppressed by GCs in a manner that could lead to an augmented cellular response. Furthermore, GCs have an impaired ability to inhibit NF-kB in the hippocampus and fail to induce some of their normal antiinflammatory targets like IL-1ra and IkBa. In rats, this led to an impaired ability of GCs to inhibit NF-kB activation (and striking increases in NF-kB caused by intermediate GC doses). In mice, NF-kB was activated both by GC treatment and endogenous GR signaling in neurons and myeloid cells alike. Thus, while GCs do not appear to increase pro-inflammatory signaling molecules directly, and even retain some of their expected anti-inflammatory effects on these molecules, they do suppress anti-inflammatory signaling molecules and have stimulatory effects on NF-kB. Together, these effects of GCs on the key elements of the inflammatory response to injury help to narrow the possible mechanisms underlying the GC-augmented inflammatory responses described in chapter 2. Having identified some of the GC-augmenting effects on inflammation amidst some of their expected anti-inflammatory effects, it was next important to determine whether these unusual effects had any functional significance for neuron survival. The next chapter addresses the issue of whether these pro-inflammatory effects of GCs have consequences for neuron survival during the two different acute CNS injuries. 40 Chapter 4: GC-Endangerment of Neurons Requires GC-Augmented Inflammation 4.1 Results To see if GC-augmented inflammatory responses (and if GC effects on immune cells in general) contribute to neuron death, the damage resulting from each of these injuries was measured. The effects of knocking out the GR in myeloid cells and neurons were measured after KA and MCAO in the MGRKO and FBGRKO mice. In the rat KA model, the same drugs indomethacin and minocycline were given to see if they could protect neurons from GCendangerment. 4.1.1 GC-endangerment requires GC-augmented inflammation The pro-inflammatory GC effects described in chapters 2 and 3 suggested that GCaugmented inflammation might actually be detrimental to neuron survival (58), so it was next measured whether anti-inflammatory drug treatments were more protective in the presence of GCs (Fig. 15a). To quantify neuron death, the damaged area in the CA3 and CA4 hippocampal fields was measured in tissue sections subsequent to those stained in Fig. 8 (Fig. 15b–d). In agreement with previous examples of GC endangerment, high GCs significantly worsened CA3 neuron death (Fig. 15b,c). Indomethacin was unable to protect CA3 neurons from GC endangerment, but minocycline was robustly protective. This result is consistent with the differing ability of these drugs to block the cellular response to injury and further supports the hypothesis that GC-augmented inflammatory responses contribute to and are necessary for GC endangerment. 4.1.2 GR signaling in myeloid cells endangers neurons in mice Next, the effects of GCs on neuron death were measured in the mouse injury models (Fig. 16). Several representative images (Fig. 16b,f) show that most KA-damage was restricted to the CA3 region, but GC-treatment frequently extended the damage to include CA1 neurons. Representative TTC sections (Fig. 16d,h) revealed significant cortical damage with some GCtreated mice having damage extending into the striatum. Quantification of this damage found that GC-treatment nearly doubled neuron death in both injury models. MGRKO mice were protected 41 from this GC endangerment, suggesting that GR signaling in immune cells is necessary for GCs to make more neurons die (Fig. 16c,e). Thus, despite differences in the pathology and types of immune cells responding to these two injuries, deleting GR in myeloid cells prevented GCendangerment. To see if the protective effects of GR deletion were myeloid cell-autonomous, the effects of GCs were next measured in FBGRKO mice where GR is deleted in the neurons throughout the injured brain regions. In contrast to MGRKO mice, FBGRKO mice were still susceptible to GCendangerment in both injury models (Fig. 16g,i). Removing GR from forebrain neurons also had a trend towards a protective effect post-MCAO. Together, these findings suggested that GR signaling in myeloid cells but not neurons is required for GC-endangerment. Given the protective effects of GR deletion in immune cells, we next tested whether increased GR levels are sufficient to worsen neuron death. Despite the increased GR levels, overexpressing GR alone was not sufficient to increase neuron death in either injury model (Fig. 17). These results support a pathological role for endogenous GR signaling in neurons postMCAO, and a pathological role for exogenous GR signaling in immune cells. 4.1.3 GR signaling affects BDNF levels differently in the hippocampus and cortex We next measured levels of BDNF at 12 and 72 h post-KA and at 24 h post-MCAO to see if GC treatments were affecting the production of this neurotrophin. GCs are reported to suppress BDNF levels, so this served as a positive control for a non-inflammatory system that GCs are supposed to affect to make sure that the molecular genetic manipulations could detect a similar effect. GCs suppressed BDNF levels in almost all groups as expected (Fig. 18). In the hippocampus post-KA, where BDNF levels were an order of magnitude higher than in the cortex, GCs suppressed BDNF levels in MGRKO mice but not in FBGRKO mice (Fig. 18a,b). Indeed, FBGRKO mice had lower BDNF levels in general, indicating that GR signaling in neurons is necessary for BDNF expression, but is also necessary for GCs to suppress BDNF. In contrast, in the cortex post-MCAO, GCs suppressed BDNF levels in FBGRKO mice but not in MGRKO 42 mice (Fig. 18c,d). FBGRKO mice also had higher BDNF levels in general, indicating that GR signaling in neurons reduces BDNF levels in the cortex, but it is GR signaling in myeloid cells that is necessary for GCs to suppress BDNF levels. Together, these results demonstrate opposing neuronal regulation of BDNF levels by endogenously released GCs signaling through neuronal GR depending on the brain region and furthermore that GC treatment suppresses BDNF levels via separate mechanisms in these different brain regions. 4.1.4 Endothelial cell GR is also detrimental to neuron survival post-injury Finally, the pathology of stroke is considerably exacerbated by the edema that occurs in the confined skull. The shifts in osmotic balance that lead to increased water uptake can be cytogenic or vasogenic in nature depending on the type of ischemic episode that occurs and whether it involves a disruption of BBB integrity. One potential reason why GCs might be considered to be beneficial is their reported ability to tighten the BBB, thus reducing edema. The role of endogenous GCs released post-stroke in affecting the BBB and neuron death is less clear, however, and these investigations tested whether GR signaling in endothelial cells might be an additional contributing factor to the endangering effects of GCs post-MCAO. To determine the role of GR signaling in endothelial cells during stroke, the floxed-GR and overexpression mouse lines were crossed to the Tie2-CRE driver (EGRKO and EGROV). This promoter expresses in endothelial cells, myeloid cells, and the female germline. The female germline expression resulted in the serendipitous generation of a deleted version of the floxed GR allele (Fig 19a,b). This null allele was used to improve the recombination efficiency by placing the floxed allele over the deficiency (Fig. 19a). “WT” mice were thus hemizygous for GR expression in these tissues. To verify the endothelial cell expression of this promoter, it was crossed to the ROSA-lacZ reporter (Fig. 19c). These mice exhibited a similar staining pattern to mice where the Tie2-CRE was crossed to the overexpression mice with the GFP reporter of rtTA expression (Fig. 19d). Finally, to determine whether GR protein levels were actually altered by our manipulations, blood microvessels were isolated from the brains of WT, EGRKO, and EGROV mice and GR levels were assayed by western blot (Fig. 19e). These experiments 43 demonstrated efficient overexpression of the GR in endothelial cells of EGROV mice and a strong trend towards a decrease in EGRKO mice. Having demonstrated the efficacy of the GR manipulations in EGRKO and EGROV mice, the amount of neuron death post-MCAO was measured. Relative to their hemizygous WT littermates, EGRKO mice had smaller infarcts post-stroke (Fig. 20a). In a similar manner, EGROV mice had larger infarct. Due to low fecundity insufficient EGRKO mice could be obtained to measure the effects of exogenous GC treatment; however, such treatment in EGROV mice was particularly detrimental to neuron survival (Fig. 20b). To see if GCs were having their expected effects on molecules that comprise the BBB, their effects on occludin and eNOS levels were measured 24 h post-MCAO. EGROV mice had no differences in either of these proteins (Fig. 20d,f), but EGRKO mice had increased occludin levels and a trend towards increased eNOS levels (Fig. 20c,e). Together, these results suggested that endogenous GR signaling in endothelial cells is detrimental to neuron survival and suppresses occludin expression after MCAO. 4.2 Discussion 4.2.1 GC effects on inflammation contribute to GC endangerment of neurons These experiments determined that the effects of GCs on inflammatory cells are not only detrimental to neurons, they are required for GC endangerment to occur. Several of the molecular explanations described in chapter 3 suggested the possibility of this outcome. The reduced ability of GCs to inhibit pro-inflammatory signals in the hippocampus like IL-1beta could have particularly deleterious consequences for neurons because the increased neurotoxicity of microglia lacking CX3CR1 is mediated via IL-1b (115). Furthermore, NF-kB activation in immune cells during excitotoxicity increases neuron death (102) and GCs had an impaired ability to inhibit NF-kB and in many instances increased NF-kB activation. To see if GC-augmented inflammation might actually worsen neuron death, we tested whether the anti-inflammatory effects of minocycline could protect neurons where GCs could not. 44 Indeed, treatment with minocycline blocked GC endangerment, arguing that GCaugmented inflammation is necessary for GCs to endanger neurons. Fittingly, indomethacin did not reduce immune activation and was also unable to protect neurons. GC-augmented inflammation alone is not sufficient to endanger neurons, however, because minocycline also suppressed cellular responses in intact rats but was not neuroprotective (compare Fig. 8b and Fig. 15c). The fact that decreasing the cellular response in the context of high-GCs was protective, argues that GCs either make neurons more vulnerable to the potential toxicity of an augmented inflammatory response or they increase the toxicity of that response itself. Minocycline can also have anti-apoptotic effects, but GCs do not endanger neurons by increasing apoptosis (121), making this an unlikely explanation for the effectiveness of minocycline. Thus, GC-effects on inflammation are likely to contribute, at least in part, to GC endangerment. This fits with the literature describing several different ways that GCs have been shown to imperil injured neurons. During the energy crisis caused by excessive excitatory neurotransmitter release, GCs impair multiple processes including glucose transport, glutamate re-uptake, calcium sequestration, and the scavenging of reactive oxygen species (97). None of these effects can individually account for GC endangerment, supporting the notion that GC endangerment emerges from the varied effects of these hormones. These findings add a surprising new angle to GC endangerment, since the anti-inflammatory properties of GCs might have normally been expected to counteract some of their endangerment. Taken together, these data support the conclusion that GC-augmented inflammation in the hippocampus contributes to worsened neuron death and is not simply a consequence of GCs making more neurons die. Furthermore, microglial activation is required for GC endangerment, despite all of the alternative mechanisms by which GCs can imperil neurons. The present results demonstrate a functional role for GC-augmented inflammation in worsening neuron death. 4.2.2 GC endangerment requires myeloid cell GR, not neuron GR Deletion of the GR in myeloid cells and neurons much more clearly revealed differences in how endogenous GCs and exogenous GCs affected neuron death. Exogenous GC-treatment 45 endangered neurons after either KA or MCAO but could only be reversed by deletion of the GR in myeloid cells, and deleting GR in neurons did not confer the same protection. This suggests that the neuron-endangering effects of exogenously applied GCs are largely mediated via their effects on myeloid cells. This outcome is in contrast to the expectation that GCs endanger neurons by impairing some of the energy-intensive processes required for neuron recovery. Secondly, these results demonstrate that the endangering properties of GC-augmented inflammation are general enough to worsen neuron death from either stroke or excitotoxicity. 4.2.3 Endogenous GCs are also detrimental to neuron survival, but less so The endogenous GCs released post-injury were considerably less detrimental to neurons than the exogenous GCs (and were less activating of immune responses (Fig. 6b,c)). The recruitment of peripheral leukocytes to an injury site required endogenous GC signaling in myeloid cells post-MCAO (Fig. 6g–j), and correspondingly, endogenous GC signaling in myeloid cells had a trend towards increasing infarct volume (Fig. 16e.). These effects were mild, however, with the slightly larger endangering effects of endogenous GCs emerging from their signaling in neurons themselves. Supporting this, there were trends towards GR signaling in neurons worsening infarct post-MCAO (Fig. 16i) and towards overexpressing GR in neurons making KA injury worse (Fig. 17c). The neuron-specific endangering effects of GR signaling could involve impairment of glucose transport into energetically compromised neurons or impairment of calcium sequestration and reactive oxygen species quenching. These neuron-intrinsic effects of GR signaling would be expected to be more visible in the larger injury caused by MCAO and indeed they were (Fig. 16i). The increased KA damage from GR overexpression in neurons implicates either the energetically demanding transgene-overexpression or the GR overexpression in the increased damage due to KA. The fact that CRE-expression was not detrimental in FBGRKO mice argues against the increased damage being an artifact of transgene expression. In rats, the role of the endogenously-released GCs post-injury in mediating neuroprotection or endangerment can be roughly determined by comparing the low GC rats with the intact rats. Other studies have found a protective effect of removing GCs (63), and if intact 46 and low-GC rats are compared across all drug treatments, there is a nearly significant main effect of low-GC treatment to reduce the damage relative to intact animals (F = 3.182, df = 1, P = 0.0823). This suggests that the power in this experiment may simply be insufficient to distinguish between low and intact animals if indeed there is a difference. A post-hoc power analysis was performed to determine the n that would be required to detect such a difference. The approximate standard deviation in damage for either low or intact rats was ±15% making the difference between the observed means approximately 0.5 standard deviations in size. To be able to detect an effect this size within a 3x3 ANOVA, an n of approximately 14 mice per group would be required. Instead, each of these groups had an n ranging from 5–13 animals due to mortality (although there were no significant differences in mortality between any of the treatment groups). Thus, it is likely that endogenous GCs also endanger neurons, but the effect was much bigger when stress level GC treatments were given. 4.2.4 GR signaling has cell-autonomous effects on BDNF levels As reported in the literature, GC-treatments suppressed BDNF levels in almost every treatment group (Fig. 18). GR signaling mediated this suppression differently in the ischemic cortex and injured hippocampus. In the injured hippocampus, endogenous GR signaling in neurons was required for increased BDNF levels, and this effect was reversed by exogenous GCtreatments (Fig. 18b). In contrast, in the ischemic cortex, endogenous GR signaling in neurons suppressed BDNF levels and exogenous GC-treatments did not require neuron GR signaling to reduce BDNF (Fig. 18d). Instead, myeloid cell GR signaling was necessary for GCs to suppress BDNF levels in the infarcted hemisphere (Fig. 18c). Thus, GR signaling in neurons affects BDNF levels oppositely in the injured hippocampus and cortex. The endogenous GCs released post-injury activate BDNF expression post-KA but they suppress BDNF post-MCAO. In addition, the amount of BDNF produced by endogenous GR signaling in neurons was equivalent to the amount suppressed by exogenous GC treatments. This could mean that in hippocampal neurons, GC treatments block the induction of BDNF by endogenous GR signaling. In cortical neurons, endogenous GCs do not induce BDNF, 47 they suppress it in a fashion that works in parallel with the ability of exogenous GCs to suppress BDNF. This additional GC-mediated reduction in BDNF levels in the cortex might be mediated by GR signaling in myeloid cells. In support of this, there is a much larger immune cell response to injury in the ischemic cortex than in the injured hippocampus and BDNF levels are much lower in the cortex than in the hippocampus, perhaps lowering the threshold to make it easier for nonneuronal signaling to influence BDNF levels. Together, these results demonstrate opposing neuronal regulation of BDNF levels by endogenously released GCs signaling through neuronal GR depending on the brain region and furthermore that GC treatment suppresses BDNF levels via separate mechanisms in these different brain regions. 4.2.5 GR signaling in endothelial cells also endangers neurons during MCAO The Tie2 promoter is not ideal for studying brain endothelial cells because it also expresses in myeloid lineage cells, making specific interpretations difficult. Fortunately, this undesired non-specificity of the Tie2 promoter is likely to overlap considerably with the myeloid lineage specificity of the LysM promoter. An imperfect solution might therefore be to compare effects in MGRKO and EGRKO mice to identify differences that might be attributable to endothelial cell GR actions. Endogenous GR signaling in endothelial cells increased neuron death, and overexpressing the GR increased neuron death even further, albeit not significantly. In addition, GR overexpression was proportionally increased whether or not additional GC treatments were given. These results suggest that in endothelial cells, GR expression is a rate-limiting factor in determining neuron survival. GR overexpression was rarely able to achieve significant effects in any of the mouse lines examined, but the near-significance measured here could result from the combined effects of GR overexpression in endothelial and myeloid cells. Comparison of EGRKO and MGRKO mice post-MCAO shows that relative to their WT controls, EGRKO mice had a 30% reduction in infarct volume whereas MGRKO mice had a 10% reduction in infarct volume. This supports the conclusion that GR signaling is detrimental in both endothelial cells and 48 myeloid cells. Other plausible, but slightly less parsimonious explanations would include differences in the population size or type of myeloid cells targeted by the two different promoters, or synergistic effects of having GR removed in multiple cells exacerbating the injury beyond the contribution of each individual cell type. These results suggested that GR signaling in endothelial cells is detrimental to neuron survival in a manner that is independent from previously described mechanisms by which GCs endanger neurons. To measure the effects of GR signaling in endothelial cells, levels were measured of two key proteins regulated by GCs and involved in the integrity of the BBB and the response to ischemia, occludin and eNOS. GCs would be expected to increase occludin levels, based on their reported ability to tighten the BBB by increasing tight junction protein expression (122). Instead, at 24 h post-ischemia, occludin levels were significantly reduced by GR signaling in endothelial cells. This effect may be more specific to the endogenous GCs released during injury as opposed to the effects of exogenous GC treatments, which were not measured in this case. Finally, in contrast to the detrimental effects of increased NO synthesis by iNOS in myeloid cells, eNOS expression in endothelial cells has been reported to be rapidly increased by GR agonists during ischemia in a fashion that confers neuroprotection (123). Increased NO synthesis in the vasculature would be of particular therapeutic benefit during ischemia because it increases vasodilation and facilitates blood flow. In contrast to this expectation, the present findings indicated a trend towards GR signaling in endothelial cells suppressing eNOS, however this remains to be investigated further because the time point analyzed here was 24 h post-stroke, and eNOS is particularly important in the early time points post-stroke. Additionally, the caveats associated with GR antagonists may apply, in that they potently suppress endogenous GC release so if the drug-resistance transporters in the BBB are not overwhelmed, observed effects could be the result of a decrease in GR signaling (see later discussion in section 5.2.2). To summarize, endogenous GR signaling in endothelial cells endangered neurons via several unique mechanisms including decreased production of tight-junction proteins and 49 potentially decreased activation of a neuroprotective vasodilatory response. The inflammatory involvement in this endangerment cannot be completely ruled out. 4.3 Conclusions GC treatments are particularly detrimental to neurons post-ischemia or excitotoxicity, and even the endogenous GCs released in response to injury are detrimental to neurons. The neuron endangerment caused by exogenous GC treatments requires microglial activation and specifically GR in myeloid cells to occur. In contrast, GR effects in neurons mediated more of the detrimental effects of endogenously-released GCs. GCs suppress BDNF via different mechanisms following these injuries and the one case where endogenous GR signaling in hippocampal neurons increased BDNF levels, this same activation was suppressed by GC treatments. Finally, endothelial cell GR signaling was also detrimental via a separate set of mechanisms that likely involve reduced protective responses and compromised BBB integrity. Together, these findings refine the picture of GC endangerment, demonstrating that the circumstances that lead to increased neuron death require GC-effects on immune cells to occur, and further that endogenous and exogenous GCs differ in their capacity to endanger neurons. 50 Chapter 5: General Discussion and Conclusions 5.1 Primary hypotheses tested and interpretations 5.1.1 GCs augment inflammation To summarize the situations where GCs can increase inflammation, several tables are presented. Table 1 lists several representative examples of the literature on GC-augmented inflammation prior to this dissertation. Table 2 lists the parameters that are the most important in dictating whether GCs will increase or decrease inflammatory responses. Whether they are generally pro- or anti-inflammatory is also indicated, although these are broad generalizations and the priority of the parameters has yet to be worked out. Finally, Figure 21 summarizes the findings of this dissertation by indicating which inflammatory factors are affected by GCs during acute CNS injury. One criticism of these findings is that more neurons dying should equate with more inflammation, so it is difficult to disentangle the two when GCs are already known to endanger neurons via mechanisms that are separate from their effects on the immune system (63). The impact of this criticism is lessened by several observations about the nature of GC-augmented inflammation. First, as described, acute GC treatments can increase peripheral inflammatory responses (6), so it is not a far stretch that GCs might have similar effects in the CNS. Furthermore, in this specific injury model the pro-inflammatory effects of GCs were observed prior to the emergence of neuron death (63). This suggests that the enhanced inflammation is not simply the result of GC-increased damage. Moreover, a correlative analysis of those data indicate that the extent of enhanced inflammatory cell migration at a particular time point post-insult predicts the extent of augmented neurotoxicity at a later time point. This finding is compatible with the idea that GC-enhanced inflammation may, in part, contribute to the increased neuron death. In contrast, the extent to which GCs augment neurotoxicity at a particular time point does not predict the subsequent extent of inflammation (63). Finally, GC-enhanced neuroinflammation can occur in the absence of neuron death (i.e. from peripheral LPS administration) (85, 87). 51 The present studies also made note of the relationship between inflammation and neuron death. Whenever possible, the amount of neuron death in each individual animal was plotted against each measurement of inflammation and a regression analysis was performed. Neither CD11b, nor CD68 signal had any correlation with the amount of neuron death post-KA (measured in the next subsequent tissue section). For protein measurements like p65 where neuron death was impossible to quantify by histology in the same animal, the amount of neuron death was estimated by quantifying the amount of spectrin cleavage in the same sample. Spectrin is a cytoskeletal protein that is cleaved in stereotypical fashion by the caspases activated during neuron death (124), and initial studies demonstrated that it is a reliable marker for the size of the nissl-stained lesions post-KA (data not shown). The amount of p65 activity did not correlate with spectrin cleavage either, and these observations together supported the conclusion that inflammatory activation does not correlate with the amount of neuron death at the same time point. Because GCs are some of the most anti-inflammatory compounds known, the notion that they can increase inflammation in the CNS has met with skepticism (125). Two central issues comprised the critical response to our review article (126). The first issue concerned the misunderstanding that the literature review was attempting to argue that GCs increase brain inflammation in an uninjured animal and that this was support for the “GC cascade hypothesis.” “This review’s attempt to make glucocorticoids inflammatory appears to represent the first faltering steps to negotiate the value of the glucocorticoid cascade hypothesis, which is wholly dependent on the primary role of excessive glucocorticoids in stress-induced pathology (127). Although it has been established that acute stress-induced catecholamines can activate brain inflammatory pathways, no data is presented in this review demonstrating that stress-induced elevations of glucocorticoids can do the same, either acutely or chronically. Instead, the hypothesis derives from more complex data regarding the capacity of chronic stress to enhance brain inflammatory responses to lipopolysaccharide or kainic acid; effects that were reduced by the glucocorticoid antagonist, RU486 (125). With increasing recognition that inflammation is a 52 central mechanism for a number of diseases that are exacerbated by stress, including cardiovascular disease, diabetes, cancer and neurodegenerative disorders, the fact that glucocorticoids are generally recognized as one of the most potent anti-inflammatory hormones in the body (128) poses a major problem for the glucocorticoid cascade hypothesis; a dilemma that the authors have chosen to resolve by renegotiating the impact of glucocorticoids on inflammatory pathways. However, increasing data (largely from humans and non-human primates) suggest that, if anything, chronic stress-related disorders are associated with decreased (not increased) glucocorticoid signaling, whether it occurs at the level of the glucocorticoid receptor and/or its ligand (129). Thus, a more empirical hypothesis is that reduced glucocorticoid signaling leads to increased inflammation and thus exacerbation of disease, whether in the body or brain. In sum, this review has contorted the immunologic effects of glucocortiocoids [sic] into a pretzel in an attempt to reconcile the irreconcilable.” The problem with this argument is several-fold. First and foremost, the GC cascade hypothesis is irrelevant because it is about normative brain aging (127), and the focus of the review was acute necrotic insults and how GCs affect inflammation during those injuries. The finding that GCs can augment the inflammatory response during acute neurological injury is the much more pertinent situation because this is precisely the setting where GCs normally decrease inflammation. The criticism dismisses this scenario as “more complicated” despite agreeing that GCs have been seen to unexpectedly increase inflammation in this setting. Interestingly, a study that was not included in the review found that GR signaling can increase microglia proliferation in the brain in the absence of any injury (105), perhaps contributing to a heightened state of readiness for any subsequent immune challenge (130). Finally, the suggestion that GCs augment inflammation because of lower GC signaling is in opposition to the finding that the enhanced inflammation is prevented by blocking glucocorticoid receptors (81, 85) or GC synthesis (63). That can only occur if there are elevated GC levels, not lower than normal ones. The second issue raised was the “paucity of data supporting the authors’ hypothesis that chronic stress-levels of glucocortiocoids [sic] “can actually increase inflammation” in the 53 brain...as acknowledged by the authors, definitive mechanisms for these effects of chronic stress, including the potential impact of glucocorticoids on immune cell trafficking and neuronal toxicity (which do not represent direct effects of glucocorticoids on inflammatory processes), cannot be established without further experiments.” This argument could not be dismissed at the time, but the fact that mechanisms are not yet delineated for these observations poses an interesting area of future research, not an argument against their existence. Indeed, the rationale for repeating this published dialogue here is to emphasize the relevance of the data described in this dissertation to the key criticisms initially levied against this hypothesis. The findings summarized in chapter 2 resulted from an effort to consolidate the proinflammatory effects of GCs into a set of repeatable observations that could be manipulated experimentally to investigate their underlying mechanisms. It is also still possible that GCs are having cell-autonomous effects on neurons that result in an increased inflammatory response earlier and more neurons dying at later time points (in accord with the regression analysis mentioned previously (63)). More simply stated, this is an issue of correlation and causation. To resolve this issue more conclusively, the mouse genetic model was used where GR was deleted in neurons or myeloid cells. If stress-level GCs are increasing inflammation via signaling through the GR in myeloid cells, then it would be expected that they could not do this in MGRKO mice. To summarize the observed pro-inflammatory effects of GCs, endogenously-released GCs increased CD11b/c+ signal in rats post-KA and they increased leukocyte recruitment in a GR-dependent fashion to CNS and peripheral injuries. These findings support the hypothesis that GR signaling in myeloid cells due to endogenous GCs released post-injury actually activates them and increases their recruitment to the injury site. Exogenous GC-treatment also had proinflammatory effects on CD11b/+c signal in rats post-KA and CD68+ signal in mice post-KA, and the increased intensity of CD68 signal also required GR signaling in myeloid cells. These results also showed that GC treatment could reduce the recruitment of peripheral monocytes and likely granulocytes to the infarct post-MCAO, an effect that was myeloid GR dependent. Thus, across multiple injury models and species, GR signaling in myeloid cells increases their 54 activation and can both activate and suppress their recruitment depending on the context of GC exposure. 5.1.2 GCs suppress anti-inflammatory signals and increase NF-kB activation To begin to assess the molecular mechanisms behind these divergent GC effects, several of their most prominent targets were analyzed next. The existing literature reported mixed effects of GCs, with some reports of increased NF-kB or cytokines, some reports of no suppression, and some reports of GCs acting in an anti-inflammatory manner (Table 1). These disparate findings suggested no obvious explanations for the cellular activation profiles established in chapter 2, so a broad survey of the most likely candidates was conducted. As a first-most likely possibility, GCs might increase pro-inflammatory signaling molecules. This hypothesis turned out to be largely untrue, however, it did reveal that GCs had a diminished capacity to suppress the production of some of these inflammatory mediators. Given this diminished capacity, the ability of GCs to induce some of their common anti-inflammatory targets was measured. Indeed, GCs did not induce some of their expected anti-inflammatory targets like IL-1ra and IkBa. GCs also suppressed the production of several other antiinflammatory molecules in the rat hippocampus that restrain microglial activation and toxicity like CX3CL1, CX3CR1, and CD22 (111). In rats, the GC-mediated suppression of these anti-inflammatory genes (Fig. 10b) corresponded with the amount of cellular activation post-injury (Fig. 8b). Low-GC rats have onehalf of the cellular activation of high-GC rats and have correspondingly elevated antiinflammatory gene expression. Because high-GCs did not suppress anti-inflammatory genes beyond the level of intact rats, it could be that GC signaling is required in a permissive fashion to facilitate an appropriately robust inflammatory response. It is possible that if intact rats were given high-GC treatment that these genes might be even further suppressed since intact rats given additional GCs have greater hippocampal immune cell responses to excitotoxicity than intact rats alone (63) and indeed GC-treated intact mice had lower CX3CL1 levels and correspondingly augmented inflammatory responses to injury (compare Fig. 11b,e and Fig. 14b,e). 55 Despite widespread potentiation of p65 nuclear levels by both exogenous and endogenous GCs, one set of WT mice that were GC-treated did not have increased p65 nuclear hormone levels. This could reflect the fact that FBGRKO mice were used at an older age to ensure efficient recombination. Aged animals have elevated GC levels and could have a decreased ability to respond to exogenous GCs (72, 131), although these animals were only 4-6 months of age so these effects should not be present yet. An alternative explanation is that subtle differences in the GC dose that each group of animals was exposed to might have had an effect on the amount of p65 activity post-injury. The dose-dependent effect of GCs on NF-kB activity postKA in rats supports this possibility (Fig. 13a,b). Finally, it could be the case that there is increased NF-kB activity in these mice, but no observable difference in p65 levels in the nucleus, as the two do not necessary equate. Instead, these findings support the conclusion that GCs, particularly those released during moderate stress, can increase p65 nuclear localization and activity, and in the hippocampus GCs are less able to reduce the activation of p65 than in other tissues. Finally, one interpretive confound must be considered. Previous studies using the LysMCRE driver have found that its expression can increase in immune cells after they have been activated (102). This may matter less for the floxed GR allele based on the 70% reduction in GR protein levels in extracted, unactivated microglia, but it could still be the case that injury increases GR deletion in MGRKO mice. The half-life of GR levels is thus an important factor to consider when analyzing time-dependent effects. It is possible that CRE-expression is activated by the injury such that there is insufficient time for GR protein levels to decrease by 12 h, but there is sufficient time by 72 h leading to greater effects only at the later time point. Supporting this, GR half-life is 4 h in cultured cells (132). Together, these molecular investigations paint a more detailed picture of GC-augmented inflammatory responses as GC-mediated suppression of anti-inflammatory signaling molecules, particularly CX3CL1, accompanied by a failure of GCs to induce some of their normal antiinflammatory targets in the hippocampus post-KA. The GC-augmented inflammatory responses 56 post-MCAO are more likely to be due to effects of GCs more directly on immune cells themselves. 5.1.3 GC-augmented inflammation is necessary for neuron death All of the evidence describing the mechanisms of GC-augmented inflammation suggested that it might also contribute negatively towards neuron survival post-injury. Indeed, following either injury, GC effects on myeloid cells were required for GCs to endanger neurons. Furthermore, GR signaling largely suppressed BDNF levels and GR signaling in endothelial cells was also detrimental to neuron survival. These findings together offer strong support for GCendangerment and further characterize it as an effect that largely depends on these unusual effects of GCs on inflammatory responses in the CNS. Some of the most likely molecular mediators of this increased neurotoxic inflammatory response are the decreased expression levels of anti-inflammatory cytokines like CX3CL1 that normally keep the toxicity of microglial IL-1beta in check. Accompanied by a reduced ability of GCs to stimulate some of their normal anti-inflammatory targets in the hippocampus, these effects could contribute to neuron death post-injury. Although they regulated CX3CL1 oppositely in the cortex, the increases in this chemokine during ischemic injury are also described to have detrimental effects on neuron survival (120). Finally, the GC-augmented NF-kB activation is also likely to carry pathological consequences, as activation of myeloid cell NF-kB is detrimental during both MCAO and KA injury in mice (102). In support of this mechanism for GCendangerment via augmented inflammation, MGRKO mice were protected from GC-induced prolonged p65 nuclear translocation following both of those injuries. 5.2 Secondary hypotheses tested and interpretations In the course of studying their general effects on inflammation and neuron death, several more specific side-hypotheses were tested about some of the parameters that determine how GCs function. 5.2.1 Timing and duration of GC exposure One of the central distinguishing features that determines how GCs will function is the 57 timing and duration of their exposure relative to the injury. An acute single exposure to stresslevel GCs has been reported to stimulate both CNS and peripheral immune responses (133, 134), provided that it occurs prior to the inflammatory challenge. GC-treatments beginning 12 h prior to LPS treatment in humans potentiate plasma IL-6 and TNF-alpha levels (48, 49). One report began to see stimulatory effects on cytokine production in the CNS if GC exposure occurred 2 h ahead of peripheral LPS challenge, contrasted with suppressive effects if GCs were given 1 h after the challenge (133, 134). Indeed, several studies have found that GCs given at the same time as or following an inflammatory challenge are more likely to have classic anti-inflammatory effects (23, 62, 84). A number of these investigations looked at the effects of acute stress but not GCs. Nonetheless, many of the effects of prior acute stress match with the effects of prior GC treatment on similar measurements. Prior acute stress also has measurable effects on subsequent inflammation in the CNS. If animals are exposed to an acute stressor 24 hours prior to LPS treatment, the result is an enhanced inflammatory response across several measures, including plasma and brain levels of IL-1β and TNF-α (50). More specifically, prior acute stress was found to enhance LPS-induced production of IL-1β in microglia (133). The enhanced response in the brain is relatively long-lived, being observable for at least four days following the stressor (51). Injection of IL-1 receptor antagonist completely blocked stress-enhancement of IL-1β production, illustrating that pro-inflammatory signaling by IL-1β is necessary to observe its own potentiation by stress. In addition, centrally injected human recombinant IL-1β was sufficient to reproduce the effects of an acute stressor (135). These findings argued that acute stress and likely GCs can have stimulatory effects on immune responses provided that they occur prior to the injury, an effect that was described as GCs priming the inflammatory response to injury (130). For the present studies, a sub-acute duration of exposure to stress-level GCs was selected for several reasons. First, this duration is common in a clinical setting, lending the results additional relevance. Second, previous work in rats used this duration of exposure to increase CNS injury and inflammation (63, 87). This duration of GC exposure is short enough that GR 58 expression levels might not be decreased in compensatory fashion. This sort of receptor downregulation is normally only seen after 3 weeks of chronic restraint stress (35). For the ADX studies, GC synthesis can resume in ectopic tissues, so this timing represented a compromise to ensure that the GC manipulations were not confounded by endogenous compensatory mechanisms. In the mice, implanted GC pellets would no longer release stress-levels of the hormones if the experiment continued for many more days. Finally, it is a duration of exposure that is expected to suppress immune responses, but it begins to address the question of where the switch between the stimulatory and suppressive actions of GCs occurs (96). Thus, the sub-acute duration of GC exposure prior to the injury was more than sufficient to increase both neuron death and CNS inflammation in both rats and mice. Indeed, GC endangerment has been found to occur from GC exposure as proximally as 12 h on either side of the injury (69, 70, 106). This duration of GC exposure also began to reveal the threshold for situations where sustained exposure to GCs is supposed to suppress certain inflammatory measurements like immune cell recruitment and cytokine production. Despite these immunosuppressive effects of GC treatment, GCs also increased cellular responses and p65 nuclear activity, suggesting that their concentration dependent effects on different cell types might result in their mixed pro- and anti-inflammatory actions. 5.2.2 Synthetic versus endogenous GCs Because of their clinical relevance, the properties of synthetic GCs (most commonly dexamethasone and methylprednisone) are pervasively explored in the literature. Care must be taken when considering the actions of these compounds, as they are frequently quite different than the effects of endogenous GCs. The synthetic GC dexamethasone differs from corticosterone in that it exhibits greatly reduced binding to normal GC carrier molecules such as the corticosteroid-binding globulin, and has an enhanced in vivo affinity for GR as opposed to MR (15, 35). But an important additional difference is that dexamethasone crosses the blood-brain barrier (BBB) poorly due to the activity of the multi-drug resistance transporter p-glycoprotein (15). As a result, dexamethasone exerts a disproportionate percentage of its neuroendocrine 59 effects at the pituitary, rather than the brain. A consequence of a strong dexamethasone signal at the pituitary is a negative feedback inhibition of the adrenocortical axis, resulting in decreased secretion of ACTH and corticosterone. This means that little dexamethasone actually penetrates the brain parenchyma and systemic corticosterone secretion drops. Given that the total amount of GCs (either endogenous or synthetic) reaching the injured brain will decrease, are the proinflammatory effects of dexamethasone merely an artifact of a net decrease in GC antiinflammatory actions? This possibility has been raised (136) as a possible explanation of observed proinflammatory effects of dexamethasone (137), but it cannot be universally applicable. First, GCs can augment pro-inflammatory cytokine expression in primary brain tissue culture, a setting where issues of BBB permeability and adrenocortical negative feedback regulation are irrelevant (86). Second, a similar question has been raised concerning the effects of dexamethasone on memory consolidation and for this topic there exist many lines of evidence that support the conclusion that like corticosterone, dexamethasone is capable of exerting its effects centrally, provided that it is given in excess (138). Finally, as reviewed, corticosterone is also capable of increasing inflammation in vivo and antagonism of GR signaling blocks this effect (81, 85). Thus, synthetic GCs like dexamethasone permeate tissues and activate nuclear receptors in a way that is different than endogenous GCs. A concerted effort is needed to understand the differences between how endogenous and synthetic GCs affect inflammation in the CNS. 5.2.3 Basal/permissive levels of GCs versus stress levels The more than half-century old view of the effects of stress and GCs on immunity has been modified in two important ways. The first is the recognition that the primary effect of stress on inflammation is not suppression, but rather stimulation; this reflects the immunostimulatory effects of sympathetic catecholamines in the first few minutes of the stress-response. These findings lead to the revisionist view that GC anti-inflammatory actions are not mediating the stress-response, but rather constraining it and facilitating recovery from it. The second modification is the recognition that the early, immunostimulatory effects of 60 stress are not merely due to catecholamines, but also to basal GC levels. These findings led to the revisionist view that at the beginning of the stress-response, basal levels (or even the earliest phases of stress-induced increases in GC secretion) have permissive immunostimulatory actions; this is the inverse-U of GC function. Importantly, this revisionist view of the stimulation of immunity by GCs does not challenge the traditional view that stress levels of GCs, and the pharmacological levels of synthetic GCs used clinically, are robustly immunosuppressive. The finding that GCs can have pro-inflammatory effects in the brain might initially seem to fit comfortably in this revisionist picture, in that these GC actions would “merely” be basal, permissive effects, predominately mediated by MR occupancy at the beginning of a stressresponse. The studies reviewed and the present experiments, however, make clear that this is not the case. It is basal, not stress levels of GCs that lead to a reduced inflammatory response in the face of a neurological challenge, both in vivo and in cell culture (62, 84-86). In contrast, sustained stress, stress levels of GCs, or heavy and selective occupancy of GR all have pro-inflammatory effects on these endpoints (63, 81, 85-89). 5.2.4 Regional differences in GC effects in the brain Virtually all the reports of pro-inflammatory GC effects have examined the hippocampus or cortex (or cultures derived from them) (63, 81, 85-87). For example, GCs can have simultaneously opposing effects on the generalized CNS inflammation induced by peripheral LPS challenge; specifically GR signaling increased NFκB activation in the hippocampus and cortex, while inhibiting its activation in the hypothalamus and the heart (139). The present findings support these results because they identify many additional pro-inflammatory GC effects in these forebrain structures. In addition, within these structures, simultaneous pro- and anti-inflammatory GC actions were observed. GC potentiation of LPS-induced NFκB activation and cytokine production was more pronounced in the cortex than the hippocampus across the full physiological range of corticosterone concentrations (87). Any number of possible mechanisms could explain these regional differences including, for example, differing concentrations of GR and MR in neurons, microglia or the vascular endothelium. Understanding the mechanisms that drive these 61 regional differences is an important subject of future research. 5.2.5 Does GC-augmented inflammation and neuron endangerment occur in mice? There was some indication that GCs might work the same in mice as they did in the rat studies that comprise most of the evidence in the literature. A variety of different injury types and GC exposures demonstrate that GCs can endanger neurons in mice (140-143); however, there was a dearth in the knowledge about normal GC functions post-injury and the role of high-stress level exogenous GC treatment in these particular acute neurological injuries. This work robustly demonstrated that GC endangerment occurs in mice in vivo as it does in rats. The reported effects of GCs on neuron death from the distal model of MCAO are mixed with some reports of endangerment and some of no effect or protection (97). Thus, the observation that GC treatment also increased the infarct volume post-MCAO further supported the literature describing situations where GCs endanger neurons and demonstrated once again that GC endangerment is a generalizable phenomenon that affects multiple injury types. 5.3 Summary and Conclusions There are a wide variety of experimental approaches and parameters measured in the literature that support the idea that GCs can increase CNS inflammation, but the number of variables that differ between reports makes direct comparisons difficult. When summarized (Tables 1, 2), however, several trends emerge. Based on the literature prior to this dissertation, the classic picture of GCs as anti-inflammatory already needed to be modified in a number of ways: 1) The immunosuppressive effects of GCs during chronic stress are involved not so much in the mediation of the immune stress-response as containment of and recovery from it. 2) While catecholamines are responsible for much of the initial immune activation by the stressresponse, GCs can also play both permissive and stimulatory roles during this phase. 3) These opposing GC actions depending on the duration of exposure fit well with other examples of inverse-U GC actions; a common effect when two separate receptors (MR and GR) have different thresholds for activation by the same hormone. 4) Prior exposure to stress or GCs can result in a “priming” of the immune response to a 62 subsequent inflammatory challenge. 5) In the CNS, sub-acute and chronic exposure to GCs and stress is not uniformly antiinflammatory and there are circumstances where GCs can actually increase inflammation; this has been observed for immune cell activation, cytokine levels, and transcription factor activation in response to necrotic and inflammatory challenges. 6) Whether pro-inflammatory GC effects occur in the brain can depend upon the timing and duration of GC exposure relative to the inflammatory challenge, the use of synthetic or endogenous GCs, and even the brain region in question. Finally, integrating the investigations presented in this dissertation adds the following additional conclusions about the role of GR signaling during acute CNS injury (summarized in Fig. 21): 1) Stress-level GC exposure prior to injury increases the cellular inflammatory response to CNS injury in both rats and mice. 2) GC-augmented inflammatory responses may result from their suppression of antiinflammatory cytokine expression (CX3CL1, CX3CR1, CD22) and a failure of GCs to activate some of their normal anti-inflammatory targets (IL-1ra, IkBa, MKP-1). 3) The effects of GCs on immune cells are necessary for GCs to endanger neurons. 4) Exogenous GC treatments are more detrimental to neuron survival than the endogenous GR signaling that occurs following CNS injury. 5) Endogenous GCs endanger neurons during ischemia via GR-signaling in endothelial cells leading to a compromised BBB. These findings produce two challenges. The first is in the realm of basic science, namely to further investigate the mechanisms underlying GC-augmented inflammation and its relationship to GC-endangerment of neurons. In particular, the role of GR signaling in astrocytes during these acute neurological injuries remains to be determined. Based on the expectation that GCs should reduce glutamate re-uptake from the synapse by astrocytes, it is expected that astrocyte GR signaling would also contribute to GC endangerment. GCs have many other targets in astrocytes, however, so it is possible that this assumption is incorrect. To further delve into the molecular 63 mechanisms behind some of these diametrically opposite effects of GR, several approaches will be necessary. The establishment of a reliable indicator of these effects in primary cultured neurons would greatly facilitate these investigations. As one example, GCs can have diametrically opposite effects simultaneously in the injured cortex and hypothalamus (85). Contrasts such as these could well arise from differences in effects of GC/GR complexes upon GREs, from GC/GR interactions with other components of gene transcriptional machinery, or from mechanisms completely independent of the genome. These differences could be easily manipulated and visualized in cultured cells from the different brain regions to identify potential mechanisms that could be verified later in vivo. As another approach, chromatin immunoprecipitation for the GR from WT and KO mice could be used to identify differences in GR-responsive genes or interactions with other gene transcriptional machinery in different brain regions under the different GC treatment conditions, potentially with cell-specific resolution as well. Finally, the scope of this research should be broadened to study GR effects in different CNS cell types during chronic degenerative CNS disorders where excessive, unchecked inflammation frequently contributes to the pathology. The second challenge is one in the realm of clinical neurology. There has often been a dogma about the uniformly beneficial effects of GCs in the face of brain inflammation thanks to their peripheral anti-inflammatory effects. Yet, as long emphasized by leaders in the field, GCs are frequently ineffective in lessening post-stroke edema and, in many cases actually worsen the outcome (144); furthermore, the same seemingly paradoxical worsening is seen when GCs are used after traumatic brain injury (145). Finally, while there is increasing evidence that chronic low-grade brain inflammation can increase the likelihood of late-onset Alzheimer’s Disease, and that long-term NSAID treatment can buffer against this, long-term GC treatment has no such beneficial effects (146). There is even some very preliminary evidence that GCs can augment inflammation in humans in a pattern consistent with rodent studies. High-stress level GCtreatments prior to LPS challenge in humans potentiate plasma IL-6 and TNF-a levels (49, 147). Even pharmacological GCs like dexamethasone have been reported to induce the expression of 64 factors associated with innate immune responses (amid decreasing components of adaptive immunity) in human blood mononuclear cells (26). Additional studies of this phenomenon in humans or human cell lines are warranted. The basic science findings considered in this thesis may well have considerable clinical implications. To conclude, these investigations demonstrate a functional role for GC-augmented inflammation in CNS injury. Specifically, exposure to moderate to severe stress-levels of GCs for as little as a few days prior to hippocampal injury is sufficient to increase cellular and molecular inflammatory responses with detrimental consequences for neuron survival. These results support the idea that prior GC exposure modifies the immune environment in the hippocampus making it more toxic to injured neurons, and could reflect a unique response of the GR-rich forebrain to GCs (85, 87). The biological significance of these pro-inflammatory effects is further supported by a recent study showing that blockade of NF-kB signaling reverses the effects of chronic stress on anxiety behavior (148). The pathological influence of GC-augmented inflammation thus affects neurological processes as diverse as psychological disorders and CNS injury. 65 Methods: Animals: (Rats). Male Sprague-Dawley rats between 250–300 g (Charles River, Gilroy CA) were housed in a 12 h light–dark cycle and fed ad libitum. All rats were treated in accordance with the Stanford University Administrative Panel on Laboratory Care regulations. (Mice). Mice were housed in a 12h light 12 dark cycle with ad libitum food and water. The floxed glucocorticoid receptor and LysM-CRE drivers were a gift from Dr. Louis Muglia. CD11b-CRE mice were a gift from Dr. Jean Vacher. Tie2-CRE and CX3CR1-GFP mice were a gift from Dr. Ben Barres. CamKIIalpha-CRE mice were obtained from Jackson labs and bred into the floxedallele background. TRErGR mice were a gift from Dr. Sam Okret and were bred into the ROSArtTA line from Jackson labs until progeny were double-homozygous for both alleles. All animals used for KA experiments were male littermates between 2-4 months of age for LysM-CRE mice and 4-6 months of age for CamK-CRE mice at the time of the experiment. All mice used for MCAO were > 3 months of age. GC treatments: (Rats). Rats were anesthetized with 2% halothane and bilaterally adrenalectomized (ADX) or left intact as a control. ADX rats were given a 100 mg s.c. pellet with different corticosterone percentages mixed with cholesterol. Low-GC pellets were 15%, intermediate were 30% or 60%, and high-GC were 100% corticosterone. High-GC rats were also given daily 10 mg/kg s.c. injections of corticosterone in peanut oil (15). (Mice). Mice were given a subcutaneous corticosterone (Sigma) pellet weighing 10mg that was implanted between the scapulae using a 12ga trochar (Innovative Research of America). Vehicle animals were subjected to the same injection but no pellet was implanted. Stereotactic surgery: (Rats). Rats were anesthetized with ketamine/xylazine/acepromazine cocktail (77/7.7/1.54 mg/ kg, i.p.). Phosphate-buffered saline (PBS) or 0.06 µg of KA dissolved in PBS was injected in 1 µL at a rate of 0.2 µl/min into the dentate gyrus (coordinates: ±3.2 mm lateral, 4.5 mm posterior 66 to bregma, 3.3 mm ventral to dura). Buprenex (0.03 mg/kg, s.c.) was given as a postoperative analgesic. (Mice). Mice were given 5% isoflurane (air mixture) to induce anesthesia which was then maintained with 1.5% isoflurane. KA was injected via stereotactic surgery into the dentate gyrus of the hippocampus using the following coordinates: 2.0mm posterior and 2.1mm lateral to bregma; 1.9mm ventral to dura. A total volume of 0.2µL was infused over the course of 4 minutes for a final dose of 50 ng of KA. Buprenex was administered as a post-operative analgesic at a dose of 0.1mL of the stock concentration/animal. Histology: (Rats). Rats were transcardially perfused 72 h post-KA with 0.9% saline followed by 4% PFA. Brains were post-fixed in 4% PFA and 30% sucrose for 24 h prior to cutting 20µm frozen cryostat sections. Sections were stained with cresyl violet and 40x images of the CA3 region were acquired using an Olympus IX70 microscope and Hamamatsu digital camera. CA3 damage was calculated by dividing the remaining CA3 cell area in the KA-injected hemisphere by that in the contralateral PBS-injected hemisphere as previously described (14). Sections were air dried for 30 min, blocked in 5% normal goat serum in PBS + 0.3% Triton-X 100 (PBST) for 1 h at room temperature, and stained overnight at 4°C with antibodies for CD11b/c (1:100, Serotec) and GFAP (1:500, Dako). Sections were washed in PBST 3x for 10 min, labeled with 488 nm and 594 nm Alexa secondary antibodies (1:200, Invitrogen) in PBST for 1 h at room temperature, washed again and stored in polyvinyl alcohol. Metamorph (Universal Imaging) was used to quantify the amount of CD11b/c+ and GFAP+ area according to established guidelines for quantifying fluorescent signal (39). Slides were background corrected and the hippocampus was traced to exclude other regions and artifacts. Signal was only counted if it was 10 intensity units above background and greater than 5 pixels but less than 25 pixels in diameter. (Mice). Animals were transcardially perfused at 72h post-KA with 0.9% saline followed by 4% PFA. Brains were post-fixed and cryoprotected in 4% PFA for an hour and 30% sucrose for 24h prior to cutting several sets of representative 20µm frozen cryostat sections in intervals across 67 each hippocampus. Nissl stain and hippocampus lesion quantification were performed as previously described (63). Briefly, tissue sections were dehydrated and stained with cresyl violet and 40x images of the hippocampus were acquired on an Olympus IX70 microscope using a Hamamatsu digital camera. Total hippocampal damage was calculated as the remaining cell area in the CA3 region of the KA-injected hemisphere divided by the remaining cell area in the CA3 region of the contralateral PBS-injected hemisphere. The next serial set of representative hippocampus tissue sections was used for immunohistochemistry. Sections were air dried for 30 min and were blocked in 5% NGS in PBS + 0.3% Triton-X 100 (PBST) for 1 hour at room temperature then they were stained overnight at 4°C with antibodies for CD11b/c (1:100, Serotec) and GFAP (1:500, Dako). Sections were washed with PBST 3 times for 10 minutes each and incubated 1hr at room temperature with 488nm and 594nm Alexa secondary antibodies (1:200, Invitrogen) in PBST. Slides were mounted in PVA and images were taken at both wavelengths. Metamorph (Universal Imaging) was used to quantify the amount of CD11b/c+ and GFAP+ area adhering to guidelines for quantifying fluorescent signal (149). Slides were background corrected, the hippocampus was traced to exclude other regions and any artifacts. Signal was only counted if it was 10 intensity units above background and greater than 5 pixels but less than 25 pixels in diameter. Prior to quantifying any staining, no-primary antibody controls were imaged to calibrate the detection limits for image acquisition. These negative controls were always dark, except in injured tissue where halos around injured cells could often be seen due to increased background in the injury site. ELISAs: (Rats). Protein was prepared from dissected CA3 tissue at the various time points in NP-40 lysis buffer. Total protein was quantified using the BCA method (Pierce) and ELISAs for MCP-1, CINC-1, and IL-1β (R&D) were run as per manufacturer instructions and quantified on a Molecular Devices plate-reader. (Mice). Hippocampus was microdissected 12 or 72 h post-KA and supernatant and nuclear extracts were prepared as previously (12). Total protein was quantified using the BCA method 68 (Pierce) and ELISAs for IL-1β, IL-6, and BDNF (R&D) were run on the supernatants (cytosolic fraction) as per manufacturer instructions and quantified on a Molecular Devices plate-reader. Drug treatments (Rats). Indomethacin was injected i.p. at 10 mg/kg beginning 24 h prior to KA and every 12 h until rats were killed. Minocycline was given s.c. at 22.5 mg/kg 30 min prior to KA, and daily for the next 72 h. LPS (1 mg/kg) was given i.p. and rats were rapidly anesthetized and decapitated. The hippocampus and frontal cortex from three rats were dissected on ice, pooled, and flash frozen for later use. All drugs were purchased from Sigma (St. Louis, MO). Quantitative PCR (Rats). Total RNA was extracted using the RNEasy Plus Mini Kit (Qiagen), and was reverse-transcribed using the iScript cDNA synthesis kit (BioRad). Intron-spanning p r i m e r s u s e d w e r e : C X 3 C L 1 : G C AT G A C G A A AT G C A A C AT C , T T G G A C C C AT T T C T C C T T T G ; C D 2 2 : C T G T G G C C G T G G A G AT A G AT , A A G A G G G T T T G G G G AT G T T C ; C D 2 0 0 : T G T T T G G AT C T G G G A A G G T C , G G A G G AT G C T G G T G A C A G AT; T G F - b 1 : G C G G A C TA C TA C G C C A A A G A , C G T G T T G C T C C A C A G T T G A C ; I κ B - a : T G A G TA C C T G G A C T T G C A G A A C G , TGTAGATGCCTCTCCAAGGATGG. Quantitative PCR was performed on a BioRad PCR machine using SyBRGreen and PCR Miner software (40). Electrophoretic mobility shift assay (Rats). CA3 was microdissected 24h post-KA and nuclear extracts were prepared as previously (12). EMSA to NF-κB was performed using the gel shift assay kit from Promega as previously (41) using the consensus oligonucleotide probe (5’AGTTGAGGGGACTTTCCCAGGC-3’). Autoradiographs of non-denaturing acrylamide electrophoresis were quantified with the ChemImager detection system (Alpha-Innotech, San Leandro, CA), using several exposure times to ensure linearity of band intensities. Flow Cytometry (Mice): Microglia were extracted from adult brains as described (101). Briefly, brains were homogenized and mononuclear cells were collected from the 70/30% percoll interface, washed, and were either allowed to adhere to coverslips or stained with antibodies for flow cytometry. Loosely adherent cells were post-fixed in either acetone (CD45) or 4% PFA (CD11b) and were stained with antibodies for CD45 (Santa Cruz) or CD11b/c (Serotec) at 69 concentrations of 1:50 each. Staining for flow cytometry was performed in FACS buffer, which included sodium azide. Cells were stained with directly conjugated antibodies for CD11b-APC, CD45-FITC, and Ly6G-PE or their isotype controls (Abcam) and analyzed on an LSR 1 machine. Flow cytometry analyses were performed using FlowJo. Western blots (Mice): Antibodies for p65 (Santa Cruz), occludin, eNOS, CX3CL1, (Abcam) were used at a concentration of 1:1000. The immunoreactive density of equally-loaded lanes was quantified using ImageJ and all samples were normalized to internal beta-actin load controls. Focal Cerebral Ischemia (Mice): Mice were anesthetized as for stereotactic surgery. The MCA was exposed and permanently occluded by electrocoagulation. An incision perpendicular to the line connecting the lateral canthus of the left eye and the external auditory canal was made to expose and retract the temporalis muscle. A burr hole was drilled to expose the MCA for elevation and cauterization by cutting and retracting the dura. After surgery, the mice were returned to their cages and allowed free access to water and food. The survival rate of the animals until the end of the experiment was 100%. Infarct Size Quantification (Mice): Brains were removed 24 hours after MCAO and cut into seven 1-mm coronal brain slices (Braintree scientific), which were stained in 1% 2,3,5-triphenyltetrazolium chloride (Merck) in 0.1 mol/L phosphate buffer. Infarct volumes were measured by sampling stained sections with a digital camera (Nikon Coolpix 990, Nikon Corp), and the image of each section was analyzed by an image analyzer (Scion Image for Windows 2000, Scion Corp, Frederick, Md). The digitized image was displayed on a video monitor. With the observer masked to the experimental conditions, the contralateral hemisphere perimeter was overlapped onto the ipsilateral hemisphere to exclude edema, and infarct borders were delineated with an operator-controlled cursor. The area of infarct, which was unstained, was determined by counting the pixels contained within the outlined regions of interest and expressed in square millimeters. Infarct volumes (in mm3) were integrated from the infarct areas over the extent of the infarct calculated as an orthogonal 70 projection. All animals displayed infarcts after the occlusion procedure, which included the cortex, subcortex, and striatum, depending on the intensity of the lesion. Statistics: Significance levels * P < 0.05, ** P < 0.01, *** P < 0.001 established by 2-way ANOVA followed by Bonferroni post-hoc tests (87). Significance established by 1-way ANOVA was followed by Tukey’s multiple comparison post hoc test. All data are expressed as means ± s.e.m. All statistical tests were performed with Prism (GraphPad). Vector art was assembled in Adobe Illustrator (Adobe Systems). 71 R KO KO a b M G R G M CD68 GR OVERLAP MGRKO WT littermate GR protein level (relative to WT control) W T Fig. 1. MGRKO mice have GR deleted in the parenchymal microglia of the cortex and * 125 hippocampus. (a) Microglia from 100 MGRKO mice or WT 75 littermates isolated on a percoll gradient and 50 analyzed by western blot for GR. One WT 25 and two representative MGRKO bands are 0 shown. All bands were WT MGRKO normalized to betaactin controls for quantification. c (microglia from 5 N-terminal Ab C-terminal Ab Hippocampus CD68 mice were pooled perGR band, n = 4 bands per DAPI g e n o t y p e ) . ( b ) OVERLAP Confocal microscopy of the cortex in MGRKO mice showing examples of CD68+ cells that lack GR co-localization (top, arrowheads) and examples of CD68+ cells that still retain GR immunoreactivity (bottom, arrows). Scale bar = 10µm. (c) Epi-fluorescence microscopy of the CA3 region of the hippocampus in WT (top) and MGRKO (bottom) mice with CA3 examples of CD68+ cells that lack GR col o c a l i z a t i o n (arrowheads) and examples of CD68+ cells that retain GR immunoreactivity (arrows). Staining patterns were similar regardless of whether the GR antibody used was raised against the N- or Cterminus of the receptor. Scale bar = 35 µm. Cortex MGRKO 72 a b LysM-CRE+ ROSA-lacZ X-gal Cortex Cortex LysM-CRE+ CD68 CRE OVERLAP WT d LysM-CRE+ Hippocampus CA3 c LysM-CRE+ ROSA-lacZ X-gal CA3 Hippocampus WT CA1 DG CA3 CD68 B-gal DAPI OVERLAP WT Fig. 2. LysM-CRE is efficiently expressed in the parenchymal microglia of the cortex and hippocampus. (a) X-gal staining of the cortex in LysM-CRE+ mice crossed to a ROSA-LacZ reporter have CRE-activity (dark staining) consistent with the distribution of microglia in the cortex. LysM-CRE negative littermates have no staining (inset). Both scale bars = 1 mm. (b) Confocal microscopy of the cortex in LysM-CRE+ mice showing examples of CD68+ cells that also have CRE immunoreactivity (arrowheads) and examples of CD68+ cells that do not (arrows). Scale bar = 10µm. (c) X-gal staining of the hippocampus in LysM-CRE+ mice crossed to a ROSA-LacZ reporter exhibit increased CRE-activity in the CA3 region of the hippocampus (dark staining) compared with WT controls (inset). Both scale bars = 1 mm. (d) Magnification of the CA3 region of the next tissue section from the animal in panel c. The beta-galactosidase reporter activity co-localizes with CD68+ cells (arrowheads). WT controls do not exhibit betagalactosidase immunoreactivity (inset). Both scale bars = 100 µm. 73 a Coordinates relative to bregma: -2.9 (± 0.1) mm -1.6 (± 0.1) mm WT FBGRKO WT GR protein level (% WT control) 3mo Fig. 3. FBGRKO mice have GR deleted in neurons of the cortex GR C-term and hippocampus. (a) X-gal dMCAO staining of the cortex in * 120 * CamKIIa-CRE+ mice that were 100 crossed to a ROSA-LacZ reporter 80 have CRE-activity (blue staining) 60 throughout the cortex and 40 hippocampus at 3, 4, and 5 20 m o n t h s o f a g e . Tw o 0 representative regions of the N-term C-term hippocampus are shown with coordinates indicated relative to the bregma. (b) GR protein levels in the infarcted cortex of WT and GR FBGRKO mice 24 h postDAPI MCAO. Western blots were performed using antibodies raised against the N- or Cterminus of the GR. n = 4. All bands were normalized to betaactin controls. (c) Epi-fluorescent microscopy for GR protein in the hippocampus of FBGRKO and WT mice. GR is deleted in pyramidal neurons in FBGRKO mice by two-months of age (arrowheads), but there are still many examples of GR colocalization with these cells at this age (arrows). A second example shows more efficient deletion of the GR in the same region (bottom). WT littermates have widespread GR expression throughout the hippocampus pyramidal neurons (inset). All scale bars = 100 µm. b GR N-term 4mo FB W G T R KO FB W G T R KO 5mo c FBGRKO WT CA3 CA3 FBGRKO WT CA3 CA3 74 a Promoter-CRE GR protein level (relative to WT control) Fig. 4. Overexpression of GR in microglia ROSA-loxP-STOP-loxP-rtTA +DOX GR and neurons using the same CRE-drivers. (a) Schematic of the TRE-CMVmin-rGR interactions between MGROV WT NeuN the transgenes used to b c FBGROV rtTA-IRES-GFP FBGROV overexpress the GR. DAPI OVERLAP The CRE recombinase 250 excises a STOP ** 200 sequence allowing the ubiquitous ROSA 150 promoter to drive 100 expression of rtTA in a 50 tissue-specific manner. CA3 In the presence of 0 WT MGROV DOX, the rtTA binds to d the TRE upstream of a 10 days DOX 7 days DOX 3 days DOX No DOX CMVmin promoter to NeuN Cortex GR increase expression of DAPI OVERLAP the rat GR gene. (b) Western blot for GR in 10 days DOX microglia extracted 7 days DOX 5 days DOX 3 days DOX from the brains of WT CA1 and MGROV mice that were given DOX for 7 7 days DOX 5 days DOX 3 days DOX CA1 Cortex days (top, 3 CA3 representative bands CA3 shown). Quantification of GR levels shows that microglial GR protein levels are nearly doubled by the overexpression system e (microglia from 5 mice new KA or dMCAO new new GC pellet begin KA were pooled per-band, DOX dMCAO quant DOX quant DOX or sham DOX n = 5 bands per genotype). (c) The rtTA 8 11 10 4 6 7 9 2 3 5 Days: 1 transgene has an IRESGFP reporter so the tissue-specific expression of this transgene can be monitored. An example of this is shown for FBGROV mice where GFP expression co-localizes with NeuN neuronal labeling in the CA3 region of the hippocampus demonstrating tissue-specific rtTA expression. Scale bar = 100 µm. (d) DOX was given to FBGROV mice for different lengths of time and GR co-localization with NeuN was assessed in the cortex and CA1 and CA3 regions of the hippocampus. Several representative epifluorescent images are shown from these different brain regions after different durations of DOX-treatment. Three days of DOX visibly elevates GR co-localization with NeuN (co-localization in white) over animals not given DOX (arrows indicate examples of this). Giving DOX for 5 days increases GR signal relative to 3 days (arrowheads indicate examples of this), but further increases beyond 5 days are difficult to distinguish. Scale bars = 100 µm. (e) Based on these observations, this experimental design was used for the overexpression experiments. Fresh DOX was given every 72 h, with experimental manipulations beginning only after 5 days of DOX treatment to allow sufficient time for overexpression to occur. 75 Serum corticosterone levels ( g/dL) a 60 * WT MGRKO 50 40 30 20 10 0 Basal 0 b 2 6 24 Serum corticosterone levels ( g/dL) Time post-stress (h) 120 100 80 60 40 20 0 GC pellet: + Days post-pellet: 1 KA on day 3: – r2=0.81 *** + 3 – + 6 – + 4 + *** + 6 + – – 4 + 6 + Fig. 5. Blood GC levels in experimental model. (a) Serum corticosterone levels in WT and MGRKO mice under basal conditions or 0, 2, 6, or 24 h after 2 h of immobilization stress in the morning (n = 6). Stress stimulates endogenous GC release to almost the same extent in WT and MGRKO mice. Circadian rhythm increases in endogenous GC release can begin to be seen 6 h post-stress and are not different in MGRKO mice. (b) Serum corticosterone levels in WT mice at different times after GC pellet implantation (n = 3-5 per group). GC pellets increased serum corticosterone concentrations which decay linearly over time (R-squared = 0.8176, first three bars). KA-treatment on day 3 post-pellet does not alter this progression and even at 6 days postpellet, serum GC levels are still elevated over non-pellet animals. 76 b 250 KA CD68+ Area (% WT Control) a 200 Fig. 6. GCs enhance cellular inflammation and can both enhance and 150 suppress recruitment. (a) 100 Tissue sections subsequent to 50 those used for KA-lesion CA3 0 quantification were stained WT MGRKO c 250 KA with CD68, a marker expressed by activated 200 myeloid cells. Scale bar = 150 100 µm. Magnification 100 shows ameboid morphology 50 of activated cells (inset). P = 0.03 0 Scale bar = 20 µm. (b) WT MGRKO CD68+ area was quantified d e f P1 in the hippocampus G1 G2 following standards for stereological quantification P2 of fluorescent signal (1). n = P3 6-11 per group. GCtreatment increases CD68+ area in both WT and FSC CD45 MGRKO mice. (c) The pixel i g h intensity of the staining in Monocytes (P2) Microglia (P3) Granulocytes (P1) 4 10 1.8 the CD68+ area quantified in b. GC treatment increases ** GC treatment 1.6 8 3 Vehicle CD68 intensity in WT but 1.4 6 not MGRKO mice. The P2 1.2 4 value indicated is for the 1 1.0 significant interaction. (d–i) 2 CNS myeloid cells were P = 0.056 P = 0.009 0 0 0 isolated 24 h post-MCAO on WT MGRKO WT MGRKO WT MGRKO a percoll gradient and j ** 22.5 analyzed via flow cytometry 20.0 for expression of CD45, 17.5 15.0 LY6G, and CD11b. (d) 12.5 10.0 Events were gated based on 7.5 forward and side scatter (G1) 5.0 2.5 to eliminate debris. (e) 0.0 WT MGRKO CD11b+ cells from G1 were also gated for CD45+ signal (G2). (f) G2 cells were differentiated into three populations using the granulocyte marker Ly6G and their relative expression of CD45 (high or low). Granulocytes (P1) are Ly6G+ and CD45 low. Myeloid cells are Ly6- and were split into CD45 high (P2), which are recruited, activated mononuclear cells and CD45 low (P3) which are resting microglia. Each population was calculated as a percentage of the G2 gate. n = 7-14 per group. (g) Fold-increase in the granulocyte population (P1) in the infarcted hemisphere relative to the contralateral hemisphere. MGRKO mice have lower granulocyte recruitment than WT controls. (h) Fold-increase in the activated monocyte population (P2) in the infarcted hemisphere relative to the contralateral hemisphere. GC-treatment decreases this population in WT but not MGRKO mice. (i) Fold-decrease in the resting microglia population (P3). The percentage of resting cells is lower in the infarcted hemisphere (reflected by a number < 1). (j) Leukocyte recruitment to a peripheral air-pouch injury in WT and MGRKO littermates. n = 8 per group. WT *** CD68 Pixel Intensity (% WT Control) * GC treatment GC treatment Vehicle ** Vehicle MGRKO CD68 4 10 4 10 10 3 10 3 10 2 10 2 SSC-H: SSC-Height 1 10 SSC-H: SSC-Height 10 1 400 600 800 10 0 1000 0 ** 200 10 1 10 2 10 3 14.1 56.7 0 0 Total Leukocyte Count (x10^6) Fold increase over contralateral hemisphere 0 <FL2-H>: PE 1 10 Ly6G 0 10 2 Fold decrease relative to contralateral hemisphere SSC 10 Fold increase over contralateral hemisphere SSC 17.5 10 3 77 10 1 10 2 10 3 a Fig. 7. Use of flow cytometry to identify CNS leukocytes. GR overexpression does not alter cell-recruitment. (a) Representative sample of b the mononuclear cells unstained unstained unstained CD45 iso CD11b iso Ly6 iso isolated from WT mouse CD45+ CD45lo CD11b+ Ly6+ brains by percoll gradient processes that were used for flow CD11b+ cytometry experiments. CD45hi Ly6+ Isolated cells were allowed to adhere to coverslips and were stained for CD45 or CD11b (top) to verify their Ly6 CD11b CD45 myeloid lineage identity. GC treatment Vehicle Cells exhibited a c MGRKO MGRKO WT WT morphology consistent with P1 P2 monocytes (DAPI, middle; Brightfield, bottom). Scale bar = 30 µm. (b) P3 Representative flow cytometry histograms demonstrating the specificity of the three fluorescent antibodies used to stain for CD45, CD11b, and Ly6G. Unstained cells (red trace) exhibit little Ly6 background fluorescence. Isotype control antibodies f 2.0 Microglia (P3) d 40 Granulocytes (P1) e 20 Monocytes (P2) (blue trace) for each channel have slightly more 30 background signal than 1.5 15 unstained controls. Antigen-specific antibodies 20 1.0 10 (green trace) have peaks corresponding with the 10 0.5 5 expected cell populations. Low and high CD45 peaks 0 0.0 0 WT MGROV WT MGROV WT MGROV (left) are distinguishable from the isotype control. CD11b signal (middle) is split into a population of cells and an off-axis population of brightlystained processes that were excluded from the analysis. The Ly6+ cell population (right) is clearly distinguishable from isotype controls. (c) Representative flow cytometry results for the quantifications presented in Fig. 2 g–i. For all plots, the Y-axis is CD45+ signal and the X-axis is Ly6G+ signal. Populations in the infarcted hemisphere (top row) were normalized to the contralateral hemisphere (bottom row) as an internal control for the quantification. (d) Foldincrease in the granulocyte population (P1) in the infarcted hemisphere relative to the contralateral hemisphere. Granulocyte recruitment is not different in MGROV mice. (e) Foldincrease in the activated monocyte population (P2) in the infarcted hemisphere relative to the contralateral hemisphere. Monocyte recruitment/activation is not different in MGROV mice. (f) Fold-decrease in the resting microglia population (P3). The percentage of resting cells is lower in the infarcted hemisphere (reflected by a number < 1). n = 8-10 (d–f). Light DAPI CD45 CD11b 100 100 100 80 80 80 % of Max 40 % of Max 40 20 20 20 0 0 3 10 2 Contralateral hemisphere 0 0 10 1 10 10 <FL1-H>: FITC 1 10 58.8 25.2 10 1 10 2 <FL1-H>: FITC 10 1 69.7 10 3 20.6 10 1 10 2 10 2.59 10 1 10 2 10 3 12.4 5 1 10 2 10 <FL1-H>: FITC 10 1 3 0 10 1 10 2 10 3 10 3 21.8 69 10 1 10 2 10 3 10 3 1.04 10 2 <FL1-H>: FITC 10 1 90.2 5.58 0 0 2 0 10 10 2 <FL1-H>: FITC 10 1 90.8 10 2 0.91 0 0 1 2.52 10 10 3 10 2 <FL1-H>: FITC 10 1 88.9 10 10 3 0 1.57 10 2 0 <FL1-H>: FITC 10 1 82.1 3 10 3 2.17 4 0 0 10 3 10 10 2 0 0 3 0.34 3.7 10 2 0 2 10 3 10 3 7.61 0 Fold increase over contralateral hemisphere 10 3 Fold increase over contralateral hemisphere Infarcted hemisphere 10 10 2 <FL1-H>: FITC 10 1 3.73 91 0 0 10 1 10 2 10 3 Fold decrease relative to contralateral hemisphere 10 1 0 CD45 60 60 60 % of Max 40 Light DAPI 78 0 10 1 10 2 10 3 a Vehicle KA 1 week 3 days Indomethacin Minocycline Indomethacin 3 days Minocycline CD11b/c Intact b Intact = sham surgery Low GC = ADX+15% High GC = ADX+100% High GC Low GC CA3 CD11b+ area (% intact control) c d * 150 ** * Intact Low GC High GC n.s. 100 50 0 Vehicle Vehicle Indomethacin Indomethacin Minocycline Minocycline Intact GFAP High GC Low GC CA3 GFAP+ area (% intact control) e *** 150 Intact Low GC High GC 100 50 0 Vehicle Indomethacin Minocycline 79 Fig. 8. Minocycline reverses GCaugmented immune cell activation in the hippocampus. (A) Experimental design: GC manipulations were performed three days prior to KA, and brains were analyzed three days later. Indomethacin was injected every 12 h starting 24 h before KA. Minocycline was injected every 24 h starting 30 min before KA. n = 5–13 per group. (B) Representative images of CD11b/c signal in the hippocampal CA3 region 72 h post-injury. (C) The total amount of CD11b/c+ area was calculated according to established guidelines for quantifying fluorescent signal (see methods). High GCs increase CD11b/c+ area relative to low GCs (* P < 0.05) and this is reversed by minocycline (** P < 0.01). (D) Representative images of GFAP signal in the hippocampal CA3 region 72 h postinjury. (E) Quantification of GFAP+ area in the same tissue sections. Minocycline treatment reduces GFAP signal across all treatment groups (*** P < 0.001). Scale bars = 500 µm, 100 µm inset. a Intact = sham surgery KA b CCL2 concentration (pg/ g total protein) Low GC = ADX+15% High GC = ADX+100% 1 week 3 days CINC-1 concentration (pg/ g total protein) c 100 CCL2 80 Dissection times (h) 2 4 8 12 18 24 48 Intact Low GC High GC 60 40 20 0 1.2 CINC-1 1.0 0.8 0.6 0.4 0.2 0.0 0 4 8 12 16 20 24 28 32 36 40 44 48 Time after kainic acid (h) Fig. 9. GCs do not increase cellular inflammation by enhancing chemokine production. (A) Experimental design: CA3 tissue was collected at 2, 4, 8, 12, 18, 24, and 48 h post-KA injury. n = 3–6 per group. (B) CCL2 production peaks between 18-24 h post-KA and is unaffected by GC treatment. (C) CINC-1 production peaks at 8 h post-KA and is also unaffected by GC treatment. 80 * CX3CL1 CX3CR1 CD22 ct Lo H wG ig C h G C 0 e 150 NF- B n.s. 100 50 0 1.5 I B 1.0 n.s. 0.5 0.0 t Lo H wG ig C h G C 10 d ac ** * CD200 TGF-beta IL-1ra In t IL-1 20 In ** mRNA fold increase relative to intact control * 3 days Intact Low GC High GC t Lo H wG ig C h G C 30 1 week ac 40 ta hippocampus IL-1 (pg/mg total protein) c qPCR In t 6 5 4 3 2 1 0 p65 nuclear activity (% WT control) b Intact = sham surgery Low GC = ADX+15% High GC = ADX+100% mRNA fold increase (relative to intact control) a Fig. 10. GCs suppress anti-inflammatory gene expression. (A) Experimental design: Rats were treated with GCs and the hippocampus was dissected at the time when the injury would normally be given. n = 5–6 per group. (B) Anti-inflammatory gene expression in the uninjured hippocampus following each GC treatment, expressed as fold-increase relative to intact. GCs suppress CX3CL1, CX3CR1, and CD22 expression (* P < 0.05, ** P < 0.01). (C) IL-1beta protein levels in the uninjured hippocampus following each GC treatment. Low and high GCs have higher IL-1beta levels than intact. (* P < 0.05, ** P < 0.01). (D) Quantification of EMSA autoradiographs for the p65 subunit of NF-kB in the uninjured hippocampus. Basal NF-kB activation is not affected by GC-treatments. (E) Quantitative PCR of IkBa in the hippocampus of uninjured rats. IkBa expression is unchanged by GC-treatments. 81 a MGRKO WT Vehicle CX3CL1 Vehicle GC treatment GC treatment 12 h post-KA 72 h post-KA 12 h * 150 120 90 * 60 P = 0.015 P = 0.135 12 h GC treatment 72 h f 90 60 P = 0.332 WT MGRKO GC treatment 150 120 90 * 60 P = 0.362 WT FBGRKO WT FBGRKO 12 h post-KA 24 h 210 dMCAO 180 150 *** 120 *** CX3CL1 protein level (% WT control) 180 P = 0.241 *** 120 Vehicle 210 KA 0 150 GC treatment Vehicle FBGRKO WT Vehicle 180 0 WT MGRKO WT MGRKO d CX3CL1 protein level (% WT control) 210 dMCAO CX3CL1 protein level (% WT control) 180 CX3CL1 24 h *** 210 KA 0 e c 72 h *** CX3CL1 protein level (% WT control) b 90 60 0 P = 0.561 WT FBGRKO Fig. 11. GCs affect CX3CL1 levels oppositely in the hippocampus post-KA and cortex postMCAO. (a) Western blot for CX3CL1 in the injured hippocampus at 12 and 72 h post-KA in WT and MGRKO mice. Two representative bands are shown, n = 5–9 per group. All bands were normalized to beta-actin controls. (b) Quantification of CX3CL1 protein levels in the injured hippocampus 12 and 72 h post-KA in WT and MGRKO mice. GC-treatment suppresses CX3CL1 levels and MGRKO mice have significantly elevated CX3CL1 levels 72 h post-KA. (c) Quantification of CX3CL1 protein levels in the infarcted hemisphere 24 h post-MCAO in WT and MGRKO mice. GC-treatment significantly elevates CX3CL1 levels and MGRKO mice have significantly lower CX3CL1 levels. (d) Western blot for CX3CL1 in the ischemic hemisphere 12 h post-KA in WT and FBGRKO mice. Two representative bands are shown, n = 5–9 per group. (e) Quantification of CX3CL1 protein levels in the injured hippocampus 12 and 72 h post-KA in WT and FBGRKO mice. GC-treatment suppresses CX3CL1 levels and FBGRKO mice have no difference in CX3CL1. (f) Quantification of CX3CL1 protein levels in the infarcted hemisphere 24 h post-MCAO in WT and FBGRKO mice. GC-treatment significantly elevates CX3CL1 levels and FBGRKO mice have significantly lower CX3CL1 levels. 82 b a IL-1 in ischemic cortex (pg/mg protein) * 4 * 2 0 60 WT MGRKO * GC treatment Vehicle 40 30 ** 20 10 0 WT WT MGRKO 150 iNOS protein levels (% WT control) MGRKO WT iNOS 125 100 75 50 25 M W G T R KO FB W G T R KO 0 f 3 * WT WT FBGRKO dMCAO GC treatment Vehicle * 15 10 ** 5 ** 0 WT MGRKO 20 WT FBGRKO FBGRKO 6 25 KA 50 9 0 d WT FBGRKO GC treatment Vehicle 12 GC treatment Vehicle 6 dMCAO WT MGRKO 150 125 WT MGRKO WT FBGRKO FBGRKO COX-2 ** 100 75 50 25 0 M W G T R KO FB W G T R KO e 8 IL-6 in ischemic cortex (pg/mg protein) IL-6 in injured hippocampus (pg/mg total protein) c 15 KA COX-2 protein levels (% WT control) IL-1 in injured hippocampus (pg/mg total protein) 10 Fig. 12. GCs suppress pro-inflammatory cytokines and are necessary for COX-2 expression. IL-1beta and IL-6 levels were measured by ELISA at 12 h post-KA (a,c) and at 24 h post-MCAO (b,d). n = 5–9 per group. (a) Hippocampal IL-1beta levels in KA-treated MGRKO or FBGRKO mice and WT controls. MGRKO, but not FBGRKO mice, have higher IL-1beta levels than WT. (b) Infarcted hemisphere IL-1beta levels post-MCAO in MGRKO or FBGRKO mice and WT controls. MGRKO, but not FBGRKO mice, have higher IL-1beta levels than WT. (c) Hippocampal IL-6 levels in KA-treated MGRKO or FBGRKO mice and WT controls. MGRKO, and nearly FBGRKO mice, have higher IL-6 levels than WT. (d) Infarcted hemisphere IL-6 levels post-MCAO in MGRKO or FBGRKO mice and WT controls. GC-treatment suppresses IL-6 levels in WT mice, but not in MGRKO mice (interaction, F = 10.16, P = 0.005). MGRKO mice have lower and FBGRKO mice have higher IL-6 levels than WT. (e) Quantification of western 83 blot for iNOS in MGRKO, FBGRKO, and WT controls 24 h post-MCAO. Neither GR KO affects the levels of iNOS significantly. All bands were normalized to beta-actin controls. (f) Quantification of western blot for COX-2 in MGRKO, FBGRKO, and WT controls 24 h postMCAO. FBGRKO mice have lower COX-2 levels relative to WT. b In ta c 15 t % 30 % 60 10 % 0 In % ta c 15 t % 30 % 60 10 % 0% NF-KB (p50/65) *** Hippocampus p65 225 *** *** *** 200 175 150 125 100 75 50 25 24 h post-KA In ta 15 ct 30% 6% 100% 0% 0 In ta 15 ct 30% 60% 10 % 0% Uninjured 250 NF B p65 nuclear activity (% intact, uninjured control) a Uninjured 4 3 Hippocampus I B mRNA Intact Low GC High GC 2 1 0 SAL d I B mRNA fold increase (relative to intact control) I B mRNA fold increase (relative to intact control) c LPS 4 3 24 h post-KA Frontal Cortex I B mRNA Intact Low GC High GC ** 2 1 0 SAL LPS Fig. 13. GCs have an impaired ability to inhibit NF-kB in the hippocampus. (a) Representative EMSA autoradiograph of the uninjured hippocampus or the injured hippocampus 24 h post-KA. The NF-kB-specific band representing p65/p50 heterodimers is indicated with an arrow. (b) Densitometric analysis of the NF-kB-specific band. GCs do not affect basal NF-kB activity, but do affect the magnitude of NF-kB activation post-KA in a dose-dependent manner. n = 6 per group. (c) Quantitative PCR of IkB mRNA in the hippocampus at baseline and 2 h after LPS challenge. GCs do not significantly affect IkB expression in this brain region. (d) Quantitative PCR of IKB mRNA in the frontal cortex at baseline and 2 h after LPS challenge. GCs induce IkB in the frontal cortex. n = 6 per group (c,d). 84 a GC treatment Vehicle WT MGRKO p65 MGRKO WT 12 h post-KA 72 h post-KA 24 h post-dMCAO c 72 h 12 h 175 dMCAO 75 50 d WT MGRKO WT MGRKO Vehicle WT p65 FBGRKO GC treatment Vehicle 150 125 *** ** * 100 * * p65 nuclear activity (% WT control) 125 *** p65 nuclear activity (% WT control) 150 KA 0 24 h *** b 100 75 * 50 0 WT MGRKO GC treatment WT FBGRKO 12 h post-KA 24 h post-dMCAO 12 h 150 KA 125 *** 100 * 75 50 0 WT FBGRKO f p65 nuclear activity (% WT control) p65 nuclear activity (% WT control) e 24 h 150 dMCAO 125 GC treatment Vehicle *** 100 75 50 0 WT FBGRKO Fig. 14. GCs increase p65 nuclear localization. (a) NF-kB nuclear localization was assayed by measuring its p65 subunit in the nuclear fraction isolated from the injured hippocampus at either 12 or 72 h post-KA or the injured cortex 24 h post-MCAO. ATwo representative bands are shown for each treatment. n = 5-6 per group. All bands were normalized to beta-actin controls. (b) Quantification of nuclear p65 levels in MGRKO mice post-KA. GC-treatment potentiates p65 activity in WT mice at both times and MGRKO mice at 12 h but after 72 h p65 activation is truncated in MGRKO mice. MGRKO mice alone have reduced p65 levels at both time points. (c) Quantification of nuclear p65 levels in MGRKO mice at 24 h post-MCAO. GC-treatment potentiates p65 activity in WT mice but not in MGRKO mice. MGRKO mice again have lower p65 levels relative to WT mice. (d) NF-kB activity was assayed in FBGRKO mice in a similar manner. (e) Quantification of nuclear p65 levels in FBGRKO mice 12 h post-KA. GC-treatment potentiates p65 activity in WT and FBGRKO mice alike. FBGRKO mice have reduced p65 levels relative to WT littermates. (f) Quantification of nuclear p65 levels in FBGRKO mice 24 h postMCAO. GC treatment does not increase p65 nuclear localization, but like MGRKO mice, FBGRKO mice have lower p65 levels than WT mice. 85 a Intact = sham surgery Low GC = ADX+15% High GC = ADX+100% Vehicle b KA 1 week Minocycline Indomethacin 3 days 3 days Indomethacin Minocycline CA1 Intact DG CA3 High GC Low GC CA4 Damaged CA3 (%) c 70 60 50 40 30 20 10 0 Damaged CA4 (%) d ** *** * ** Vehicle Indomethacin Minocycline Vehicle Indomethacin Minocycline Intact Low GC High GC 100 80 60 40 20 0 Fig. 15. GC-augmented inflammation is necessary for GC-endangerment. (A) Experimental design as in Fig. 1A. n = 5–13 per group. (B) Representative images of Nissl-stained sections following each treatment combination at 72 h post-KA. The CA3 region has the most quantifiable damage (magnified below each image). Hippocampal sub-regions are indicated with dashed lines. Arrows point to areas of neuron loss. Scale bars = 500 µm, (200 µm, magnification). (C) Quantification of CA3 lesioned area 72 h post-injury. High GCs significantly increase CA3 damage relative to low GCs (** P < 0.01). Indomethacin does not protect from this (* P < 0.05) but minocycline does (*** P < 0.001, ** P < 0.01). (D) Quantification of CA4 lesioned area 72 h post-injury. No treatments affect the widespread damage in this region. 86 KA or dMCAO 24 h dMCAO infarct quantification GC treatment GC treatment Vehicle WT MGRKO CA1 WT c MGRKO DG CA3 WT MGRKO Vehicle FBGRKO WT WT MGRKO GC treatment treatment GC WT e g FBGRKO CA1 DG CA3 60 50 40 30 20 10 0 35 dMCAO 30 25 20 15 10 5 0 60 50 40 30 FBGRKO WT FBGRKO 20 10 0 P = 0.743 WT FBGRKO 60 dMCAO 50 40 30 *** Infarct Volume (mm3) WT GCtreatment treatment GC * i Vehicle P = 0.085 WT MGRKO 80 KA 70 CA3 h P = 0.061 WT MGRKO ** f GC treatment Infarct Volume (mm3) Vehicle % hippocampus damaged d GC treatment Vehicle ** CA3 80 KA 70 * b KA lesion quantification *** 3 days GC pellet or vehicle 72 h % hippocampus damaged a 20 10 0 P = 0.480 WT FBGRKO Fig. 16. GC-endangerment requires GR signaling in myeloid cells but not neurons. (a) Experimental setup: WT and KO mice were given an s.c. 10 mg GC pellet and three days later subjected to either stereotactic infusion of KA or the distal model of MCAO. Mice were killed 72 or 24 h post-injury. n = 5-12 per treatment group. (b) Representative Nissl-stained hippocampal images from WT and MGRKO mice with or without GC pellets. Scale bar = 200 µm. The CA3 region of the hippocampus is magnified on the bottom. Scale bar = 50 µm. Arrows indicate damaged neurons. (c) Quantification of the damaged hippocampal area. GC-treatment doubles KA-induced hippocampal damage in WT mice but not in MGRKO littermates. (d) Representative TTC stained tissue sections post-MCAO from MGRKO mice with or without GC pellets. The white infarcted region is outlined. (e) Quantification of the infarct volume. GC-treatment increases infarct volume in WT but not MGRKO littermates. (f) Representative Nissl-stained hippocampal images from WT and FBGRKO mice with or without GC pellets. Scale bar = 200 µm. The CA3 subfield of the hippocampus is magnified on the bottom. Scale = 50 µm. Arrows indicate damaged neurons. (g) Quantification of damaged hippocampal area. GC-treatment increases KA-induced hippocampal damage in both WT and FBGRKO mice. (h) Representative TTC stained tissue sections post-MCAO from WT or FBGRKO mice with or without GC pellets. (i) Quantification of infarct volume. GC treatment increases MCAO infarct in both WT and FBGRKO mice. 87 40 20 0 WT 100 KA 80 d GC treatment Vehicle * 20 0 30 20 10 WT MGROV 70 dMCAO 60 50 GC treatment Vehicle 40 30 20 10 0 WT FBGROV GC treatment Vehicle 40 0 MGROV 60 40 Infarct Volume (mm3) 60 50 dMCAO Infarct Volume (mm3) 80 GC treatment Vehicle *** % hippocampus damaged 100 KA ** c b *** % hippocampus damaged a WT FBGROV Fig. 17. GR overexpression has minimal effects on neuron death. (a) Quantification of the damaged hippocampal area as in Fig. 1 in WT and MGROV mice 72 h post-KA. GC-treatment increases KA-induced hippocampal damage irrespective of genotype. (b) Quantification of the infarct volume in WT and MGROV mice 24 h post-MCAO. GC-treatment increases infarct volume irrespective of genotype. (c) Quantification of damaged hippocampal area in WT and FBGROV mice post-KA. GC-treatment increases KA-induced hippocampal damage in WT but not FBGROV mice. (d) Quantification of infarct volume in WT and FBGROV mice post-MCAO. GC treatment increases MCAO infarct irrespective of genotype. 88 1200 400 WT 300 * 200 100 P = 0.077 WT MGRKO * 4000 2000 MGRKO 400 dMCAO 0 6000 d BDNF in ischemic cortex (pg/mg protein) 0 GC treatment Vehicle P = 0.12 WT FBGRKO 0 400 dMCAO 300 ** 200 100 ** BDNF in ischemic cortex (pg/mg protein) c 800 8000 KA * 1600 KA BDNF in injured hippocampus (pg/mg total protein) b ** BDNF in injured hippocampus (pg/mg total protein) a * * 0 WT FBGRKO Fig. 18. GCs suppress BDNF levels in the hippocampus and cortex in a cell-specific manner. BDNF levels were measured by ELISA at 12 h post-KA (a,b) and at 24 h post-MCAO (c,d). n = 5–9 per group. (a) Hippocampal BDNF levels in KA-treated WT and MGRKO mice. GC treatment reduces BDNF and does not require myeloid GR signaling for this to occur. (b) Hippocampal BDNF levels in KA-treated WT and FBGRKO mice. GC treatment requires neuron GR signaling to suppress BDNF levels. FBGRKO mice have lower BDNF levels than WT. (c) Infarcted hemisphere BDNF levels post-MCAO in WT and MGRKO mice. GC treatment reduces BDNF levels in WT but not MGRKO mice. (d) Infarcted hemisphere BDNF levels post-MCAO in WT and FBGRKO mice. GC treatment suppresses BDNF levels in both WT and FBGRKO mice. FBGRKO mice have higher BDNF levels than WT. 89 a b ATG GR genomic locus: 5’UTR Exon1C loxP Floxed GR allele: 5’UTR EcoRV SpeI ExonIC SpeI 700bp SpeI 1kb Tie-2-CRE+ :: floxed GR / Tie-2-CRE- :: floxed GR / Tie2-CRE+ rtTA-GFP Lectin WT Merge EGRKO 200 WT EGROV * 150 100 50 0 EG WT R KO DAPI WT EG WT R O V e d GR protein level (relative to WT control) EGRKO: WT littermate: 5’UTR X-gal loxP ATG loxP Null allele: c $ Fig. 19. Endothelial cell KO and OV mice have higher GR levels in endothelial cells. (a) Schematic of the three different alleles at the GR locus that were used in the creation of EGRKO mice. Tie2-CRE deletes in the female germline leading to the generation of a null allele at the GR locus. To improve recombination efficiency, the floxed-GR allele was placed over this deficiency so that only one recombination event had to occur for the GR to be deleted. (b) DNA agarose gel electrophoresis of PCR on genomic DNA from WT, floxed, and null mice using primers that span the deleted region. The expected size of the deletion-spanning PCR product is ~700bp (arrow). (c) X-gal staining of the hippocampal fissure in Tie2-CRE+ mice crossed to a ROSA-LacZ reporter have CRE-activity (dark staining) consistent with blood vessel morphology. Scale bar = 10 µm. (d) Epifluorescence microscopy of the hippocampal fissure in EGROV mice. The overexpression GFP reporter (green, arrows) co-localizes with the blood vessel label lectin (red). Scale bars = 30 µm. (e) Western blot for GR on extracted blood vessels from the brains of EGRKO and EGROV mice. EGROV mice have higher GR levels than WT littermates after both were treated with DOX for 7 days. n = 7–9. EGRKO mice have a trend towards lower GR levels. n = 4–5. 90 Infarct Volume (mm3) 50 dMCAO 40 30 20 10 0 c * b 80 Infarct Volume (mm3) a EGRKO P = 0.057 40 *** 20 0 d 100 50 0 e WT 250 eNOS Protein Levels (relative to WT) WT EGRKO EGRKO 200 150 100 50 WT EGRKO EGROV WT EGROV 200 150 100 50 0 f WT EGROV WT EGROV 250 eNOS eNOS P = 0.1 0 Occludin Protein Levels (relative to WT) 150 WT 250 Occludin eNOS Protein Levels (relative to WT) Occludin Protein Levels (relative to WT) 200 Occludin * GC treatment Vehicle 60 WT EGRKO WT dMCAO 200 150 100 50 0 WT EGROV 91 Fig. 20: Endothelial cell GR signaling also worsens infarct post-MCAO. (a) Quantification of infarct volume as in Fig. 1, 24 h post-MCAO in WT and EGRKO mice. EGRKO mice have a smaller infarct than WT littermates. n = 7. (b) Quantification of infarct volume in WT and EGROV mice. GC treatment increases infarct volume and EGROV mice also have more damage than WT controls. n = 8–10 vehicle, 3–5 GC-treated. (c) Quantification of western blot for occludin levels in EGRKO mice 24 h post-MCAO. EGRKO mice have higher occludin levels than WT mice. n = 3–5. (d) Quantification of western blot for occludin levels in EGROV mice 24 h post-MCAO. GR overexpression does not increase occludin levels. n = 4. (e) Quantification of western blot for eNOS levels in EGRKO mice 24 h post-MCAO. There is a trend towards increased eNOS levels in EGRKO mice. n = 3– 5. (f) Quantification of western blot for eNOS levels in EGROV mice 24 h post-MCAO. GR overexpression does not increase eNOS levels. n = 4. Excitotoxicity (KA) Hippocampus BBB Microglia/Macrophages EXO ENDO ENDO EXO (ENDO) CD11b/c GR EXO ENDO Neuron Death GR EXO ENDO NF-KB IL-1B IL-6 Blood Brain EXO Peripheral Leukocytes CD68 GR CX3CR1 ENDO EXO CD45 EXO ia l CD22 EXO ENDO En NF-KB dot hel GR BDNF cel ls CX3CL1 EXO ENDO Exogenous GCs = EXO Endogenous GCs = ENDO Stroke (dMCAO) Cortex BBB Microglia/Macrophages Blood Brain EXO ENDO ENDO GR NF-KB IL-1B CX3CL1 (in neurons) GR Peripheral Leukocytes IL-6 EXO ENDO ENDO Occludin (eNOS) ls GR CX3CL1 GR GR cel Neuron Death dot hel ial EXO ENDO COX-2 ENDO EXO (ENDO) NF-KB BDNF En GR ENDO EXO EXO ENDO ENDO Astrocytes GR Fig. 21. Summary of endogenous GC and exogenous GC effects during different injuries. Exogenous GCs and endogenous GCs have often-opposing effects on parameters post-injury that could affect inflammation and neuronal survival. The effects of the endogenous GCs released post-injury are indicated as “ENDO” whereas the effects of exogenous GC treatments are indicated as “EXO.” Effects that were trends but had not yet established significance are indicated in grey parentheses. For effects that are dependent on GR signaling in a particular cell type, an open, dashed arrow indicates this relationship and the stimulatory or inhibitory effects of endogenous and exogenous GCs is indicated. Stimulating effects are indicated by arrows and inhibitory effects are indicated by blunt-headed arrows. Microglia/Macrophages EXO (ENDO) EXO ENDO ENDO NF-KB IL-1B IL-6 EXO ENDO EXO ENDO CD11b/c GR EXO 92 Blood GR Peripheral Leukocytes CD68 GR CX3CR1 CX3CL1 CD45 EXO GR ells Neuron Death BBB Brain CD11b/c PERIPHERAL GC dose Acute stressor GC treatment time Concurrent Basal cort levels Concurrent Dexamethasone Peripheral Location Human PBMCs ex vivo, THP-1 cell line Mouse macrophage cell line Pro-inflammatory effect NFkB activity Inflammatory stimulus None Reference (Bierhaus et al. 2003) MIF secretion None (Calandra et al. 1995) Human PBMCs ex vivo Cytokines, complement, chemokines 5-lipoxygenase None (Galon et al. 2002) None (Riddick et al. 1997) Dexamethasone Concurrent Concurrent Dexamethasone Concurrent Human PBMCs ex vivo, THP-1 cell line THP-1 cell line IL-1 beta PMA (Wang et al. 1997) Dexamethasone Concurrent THP-1 cell line NFkB activity PMA (Wang et al. 1997) Dexamethasone or prednisolone >16 hours prior Human lymphocyte cultures Proliferation Low stress cort levels Acute 2hrs prior Leukocyte migration Low stress cort levels Concurrent Rat leukocytes in vivo and Mouse leukocytes in vivo Rat lymphocyte cultures Proliferation Mitogens, x-linking (Almawi et al. 1999) Abs, or PMA +ionomycin DTH (Dhabhar et al. 1996) (Dhabhar et al. 2005) IL-2 (Wiegers et al. 1995) Low stress cort levels >6 hours prior Mouse macrophage cell line NFkB activity LPS and IFNγ (Smyth et al. 2004) Low stress cort levels >6 hours prior Mouse macrophage cell line TNF-alpha, IL-6, nitrite LPS and IFNγ (Smyth et al. 2004) Low stress cort levels >12 hours prior Human plasma TNF-alpha, IL-6 LPS (Barber et al. 1993) Reference (Madrigal et al. 2001) CENTRAL GC treatment Acute stressor GC treatment time Concurrent Central Location Rat cortex Pro-inflammatory effect NFkB activity Inflammatory stimulus None Acute stressor 24 hours prior Rat brain IL-1 beta Peripheral LPS (Johnson et al. 2002) Acute stressor 24 hours prior Rat hippocampal microglia IL-1 beta Peripheral LPS (Frank et al. 2006) CUS Chronic 9 days after Rat prefrontal cortex Microglia activation Central LPS (de Pablos et al. 2006) CUS Chronic 9 days after Rat prefrontal cortex TNF-alpha Central LPS (de Pablos et al. 2006) CUS Chronic 9 days after Rat prefrontal cortex MAPK signaling Central LPS (de Pablos et al. 2006) CUS Chronic 14 days prior Rat hippocampus, frontal cortex TNF-alpha, IL-1 beta Peripheral LPS (Munhoz et al. 2006) CUS Chronic 14 days prior Rat hippocampus, frontal cortex NFkB activity Peripheral LPS (Munhoz et al. 2006) Dexamethasone Concurrent Mouse neuronal cell line PGD2 synthase None Dexamethasone* One hour prior Mouse hippocampus TMT High stress cort levels Chronic 3 days prior Rat hippocampus TNF-alpha, TNF-beta, IL-1 beta Leukocyte migration (Garcia-Fernandez et al. 2000) (Bruccoleri et al. 1999) Kainic acid (Dinkel et al. 2003) High stress cort levels Chronic 3 days prior Rat hippocampus Microglia activation Kainic acid (Dinkel et al. 2003) High stress cort levels Chronic 24 hours prior Rat hippocampal cultures TNF-alpha, IL-1 beta Kainic acid Intermediate stress cort levels Chronic 3 days prior Rat hippocampus, frontal cortex TNF-alpha, IL-1 beta Peripheral LPS (MacPherson et al. 2005) (Munhoz in prep) Intermediate stress cort levels Chronic 3 days prior Rat hippocampus, frontal cortex NFkB activity Peripheral LPS (Munhoz in prep) Low stress cort levels Chronic 10 days Rat hippocampus, cerebellum 5-lipoxygenase None (Uz et al. 1999) Table 1. Some experimental conditions where GCs have been found to increase inflammation. The variety of experimental conditions in which GC enhanced inflammation has been observed makes definitive conclusions difficult. The top table is a non-comprehensive list of proinflammatory effects of GCs on peripheral immunity. The bottom table lists conditions where GCs have been found to enhance signs of central inflammation and is more extensive. The type of GC treatment varies between experimental groups; however broad categorizations have been assigned. Basal stress cort levels are between 1-10µg/dL, low stress cort levels are between 10-25µg/dL, and high stress cort levels are above 25µg/dL. Dexamethasone doses are roughly equivalent to high stress cort doses at 10-7M. GC treatment time refers to the time of exposure to GCs relative to a subsequent inflammatory stimulus or measurement. See chapter 1 for a more detailed description of many of these investigations. *study potentially affected by poor BBB permeability of dexamethasone (see text, section 5.2.2). Ab = antibody; cort = corticosterone (in rodent studies) or cortisol (in primate studies); CUS = chronic unpredictable stress; LPS = lipopolysaccharride; PMA is the NFκB activator phorbol 12myristate 13-acetate; THP-1 = human monocyte cell line; TMT = trimethyltin. 93 Type of GC Endogenous (corticosterone) Synthetic GC (dexamethasone) PRO ANTI Timing of GC exposure Before injury During injury After injury Before, during and after injury PRO ANTI ANTI PRO Duration of GC exposure Acute (single exposure) Sub-acute (3-7 days) Chronic (>3 weeks) PRO PRO ANTI GC concentration Basal Moderate stress High stress Supraphysiological PRO PRO* PRO ANTI Table 2: Summary of parameters that impact how GCs affect inflammatory responses. Broad generalizations are given as to whether each variable tends to cause GCs to increase (PRO) or decrease (ANTI) inflammatory measurements. Clearly this is an over-simplification and counter-examples are prevalent, but a large percentage of these counter-examples may result from unknown rules about which of these parameters has priority over the others. The * is an example of this, as moderate GCs caused by chronic mild stress are often pro-inflammatory despite the chronic nature of the exposure, which should be anti-inflammatory. This dissertation primarily addressed the effects of endogenous GCs, both basally and during sub-acute exposure before, during, and after the injury at moderate to high-stress levels. 94 References: 1. Sapolsky RM, Romero LM, & Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55-89. 2. Cannon WB (1935) Stresses and strains of homeostasis. American Journal of the Medical Sciences 189:1-14. 3. Selye H (1936) A syndrome produced by diverse nocuous agents. Nature 138:32-32. 4. Cannon WB & Pereira JR (1924) Increase of Adrenal Secretion in Fever. Proc Natl Acad Sci U S A 10:247-248. 5. Selye H (1936) Thymus and adrenals in the response of the organism to injuries and intoxications. British Journal of Experimental Pathology 17:234-248. 6. Dhabhar FS (2009) Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 16:300-317. 7. Sorrells SF, Caso JR, Munhoz CD, & Sapolsky RM (2009) The stressed CNS: when glucocorticoids aggravate inflammation. Neuron 64:33-39. 8. Black PH (2002) Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun 16:622-653. 9. Yeager MP, Guyre PM, & Munck AU (2004) Glucocorticoid regulation of the inflammatory response to injury. Acta Anaesthesiol Scand 48:799-813. 10. Munck A, Guyre PM, & Holbrook NJ (1984) Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev 5:25-44. 11. Rosenberger PH, et al. (2009) Surgical stress-induced immune cell redistribution profiles predict short-term and long-term postsurgical recovery. A prospective study. J Bone Joint Surg Am 91:2783-2794. 95 12. Tarcic N, Ovadia H, Weiss DW, & Weidenfeld J (1998) Restraint stress-induced thymic involution and cell apoptosis are dependent on endogenous glucocorticoids. J Neuroimmunol 82:40-46. 13. Elenkov IJ & Chrousos GP (2002) Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci 966:290-303. 14. De Bosscher K, Vanden Berghe W, & Haegeman G (2003) The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev 24:488-522. 15. De Kloet ER, Vreugdenhil E, Oitzl MS, & Joels M (1998) Brain corticosteroid receptor balance in health and disease. Endocr Rev 19:269-301. 16. Karst H, et al. (2005) Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci U S A 102:19204-19207. 17. Mulholland PJ, et al. (2006) A 24 h corticosterone exposure exacerbates excitotoxic insult in rat hippocampal slice cultures independently of glucocorticoid receptor activation or protein synthesis. Brain Res 1082:165-172. 18. Dhabhar FS & McEwen BS (1999) Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci U S A 96:1059-1064. 19. O'Connor KA, et al. (2003) Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res 991:123-132. 20. Deinzer R, et al. (2004) Acute stress effects on local Il-1beta responses to pathogens in a human in vivo model. Brain Behav Immun 18:458-467. 21. Bierhaus A, et al. (2003) A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A 100:1920-1925. 22. Madrigal JL, et al. (2001) Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor kappaB-mediated mechanisms. J Neurochem 76:532-538. 96 23. Goujon E, et al. (1995) Stress downregulates lipopolysaccharide-induced expression of proinflammatory cytokines in the spleen, pituitary, and brain of mice. Brain Behav Immun 9:292-303. 24. Viswanathan K & Dhabhar FS (2005) Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proc Natl Acad Sci U S A 102:5808-5813. 25. Johnson JD, et al. (2005) Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 135:1295-1307. 26. Galon J, et al. (2002) Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. Faseb J 16:61-71. 27. Reyes TM, Walker JR, DeCino C, Hogenesch JB, & Sawchenko PE (2003) Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci 23:5607-5616. 28. Dhabhar FS (2008) Enhancing versus Suppressive Effects of Stress on Immune Function: Implications for Immunoprotection versus Immunopathology. Allergy Asthma Clin Immunol 4:2-11. 29. Dhabhar FS (2002) Stress-induced augmentation of immune function--the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav Immun 16:785-798. 30. Yoshimura C, et al. (2001) Glucocorticoids induce basophil apoptosis. J Allergy Clin Immunol 108:215-220. 31. Cox G (1995) Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol 154:4719-4725. 32. Liles WC, Dale DC, & Klebanoff SJ (1995) Glucocorticoids inhibit apoptosis of human neutrophils. Blood 86:3181-3188. 33. Dhabhar FS & McEwen BS (1997) Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun 11:286-306. 97 34. Dhabhar FS, Miller AH, McEwen BS, & Spencer RL (1996) Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J Immunol 157:1638-1644. 35. McEwen BS, et al. (1997) The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Brain Res Rev 23:79-133. 36. Blecha F, Barry RA, & Kelley KW (1982) Stress-induced alterations in delayed-type hypersensitivity to SRBC and contact sensitivity to DNFB in mice. Proc Soc Exp Biol Med 169:239-246. 37. Blecha F, Kelley KW, & Satterlee DG (1982) Adrenal involvement in the expression of delayed-type hypersensitivity to SRBC and contact sensitivity to DNFB in stressed mice. Proc Soc Exp Biol Med 169:247-252. 38. Wiegers GJ & Reul JM (1998) Induction of cytokine receptors by glucocorticoids: functional and pathological significance. Trends Pharmacol Sci 19:317-321. 39. Munck A & Naray-Fejes-Toth A (1992) The ups and downs of glucocorticoid physiology. Permissive and suppressive effects revisited. Mol Cell Endocrinol 90:C1-4. 40. Wiegers GJ, et al. (1995) Glucocorticoids accelerate anti-T cell receptor-induced T cell growth. J Immunol 155:1893-1902. 41. Almawi WY, Hess DA, Assi JW, Chudzik DM, & Rieder MJ (1999) Pretreatment with glucocorticoids enhances T-cell effector function: possible implication for immune rebound accompanying glucocorticoid withdrawal. Cell Transplant 8:637-647. 42. Sterzer P, Wiegers GJ, & Reul JM (2004) Long-term in vivo administration of glucocorticoid hormones attenuates their capacity to accelerate in vitro proliferation of rat splenic T cells. Endocrinology 145:3630-3638. 43. Bernhagen J, et al. (1994) Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF). Biochemistry 33:14144-14155. 98 44. Bernhagen J, et al. (1996) An essential role for macrophage migration inhibitory factor in the tuberculin delayed-type hypersensitivity reaction. J Exp Med 183:277-282. 45. Petrovsky N, et al. (2003) Macrophage migration inhibitory factor exhibits a pronounced circadian rhythm relevant to its role as a glucocorticoid counter-regulator. Immunol Cell Biol 81:137-143. 46. Calandra T, et al. (1995) MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377:68-71. 47. Donnelly SC & Bucala R (1997) Macrophage migration inhibitory factor: a regulator of glucocorticoid activity with a critical role in inflammatory disease. Mol Med Today 3:502-507. 48. Smyth GP, et al. (2004) Glucocorticoid pretreatment induces cytokine overexpression and nuclear factor-kappaB activation in macrophages. J Surg Res 116:253-261. 49. Barber AE, et al. (1993) Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol 150:1999-2006. 50. Johnson JD, et al. (2002) Prior stressor exposure primes the HPA axis. Psychoneuroendocrinology 27:353-365. 51. Johnson JD, et al. (2002) Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun 16:461-476. 52. Johnson JD, O'Connor KA, Hansen MK, Watkins LR, & Maier SF (2003) Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol 284:R422-432. 53. Davalos D, et al. (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8:752-758. 54. Bruce AJ, et al. (1996) Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med 2:788-794. 55. Allan SM & Rothwell NJ (2001) Cytokines and acute neurodegeneration. Nat Rev Neurosci 2:734-744. 99 56. Viviani B, et al. (2003) Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci 23:8692-8700. 57. Myer DJ, Gurkoff GG, Lee SM, Hovda DA, & Sofroniew MV (2006) Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 129:2761-2772. 58. Dinkel K, Dhabhar FS, & Sapolsky RM (2004) Neurotoxic effects of polymorphonuclear granulocytes on hippocampal primary cultures. Proc Natl Acad Sci U S A 101:331-336. 59. Watanabe H, Abe H, Takeuchi S, & Tanaka R (2000) Protective effect of microglial conditioning medium on neuronal damage induced by glutamate. Neurosci Lett 289:53-56. 60. del Zoppo GJ, Becker KJ, & Hallenbeck JM (2001) Inflammation after stroke: is it harmful? Arch Neurol 58:669-672. 61. McEwen BS & Magarinos AM (2001) Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol 16:S7-S19. 62. Nadeau S & Rivest S (2003) Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J Neurosci 23:5536-5544. 63. Dinkel K, MacPherson A, & Sapolsky RM (2003) Novel glucocorticoid effects on acute inflammation in the CNS. J Neurochem 84:705-716. 64. Sapolsky RM & Pulsinelli WA (1985) Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science 229:1397-1400. 65. Tombaugh GC, Yang SH, Swanson RA, & Sapolsky RM (1992) Glucocorticoids exacerbate hypoxic and hypoglycemic hippocampal injury in vitro: biochemical correlates and a role for astrocytes. J Neurochem 59:137-146. 66. McIntosh LJ & Sapolsky RM (1996) Glucocorticoids may enhance oxygen radicalmediated neurotoxicity. Neurotoxicology 17:873-882. 100 67. Goodman Y, Bruce AJ, Cheng B, & Mattson MP (1996) Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem 66:1836-1844. 68. Olgemoller B, Schon J, & Wieland OH (1985) Endothelial plasma membrane is a glucocorticoid-regulated barrier for the uptake of glucose into the cell. Mol Cell Endocrinol 43:165-171. 69. Stein-Behrens BA, et al. (1992) Glucocorticoids exacerbate kainic acid-induced extracellular accumulation of excitatory amino acids in the rat hippocampus. J Neurochem 58:1730-1735. 70. Stein-Behrens BA, Lin WJ, & Sapolsky RM (1994) Physiological elevations of glucocorticoids potentiate glutamate accumulation in the hippocampus. J Neurochem 63:596-602. 71. Elliott EM & Sapolsky RM (1993) Corticosterone impairs hippocampal neuronal calcium regulation--possible mediating mechanisms. Brain Res 602:84-90. 72. Sapolsky RM (1999) Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol 34:721-732. 73. Reagan LP (2002) Glucose, stress, and hippocampal neuronal vulnerability. Int Rev Neurobiol 51:289-324. 74. Patel PM, et al. (1993) Effect of ibuprofen on regional eicosanoid production and neuronal injury after forebrain ischemia in rats. Brain Res 614:315-324. 75. Hara K, Kong DL, Sharp FR, & Weinstein PR (1998) Effect of selective inhibition of cyclooxygenase 2 on temporary focal cerebral ischemia in rats. Neurosci Lett 256:53-56. 76. Golde S, Coles A, Lindquist JA, & Compston A (2003) Decreased iNOS synthesis mediates dexamethasone-induced protection of neurons from inflammatory injury in vitro. Eur J Neurosci 18:2527-2537. 101 77. Nadeau S & Rivest S (2002) Endotoxemia prevents the cerebral inflammatory wave induced by intraparenchymal lipopolysaccharide injection: role of glucocorticoids and CD14. J Immunol 169:3370-3381. 78. Rivest S (2003) Molecular insights on the cerebral innate immune system. Brain Behav Immun 17:13-19. 79. Simmons CP, et al. (2005) The clinical benefit of adjunctive dexamethasone in tuberculous meningitis is not associated with measurable attenuation of peripheral or local immune responses. J Immunol 175:579-590. 80. Gomes JA, Stevens RD, Lewin JJ, 3rd, Mirski MA, & Bhardwaj A (2005) Glucocorticoid therapy in neurologic critical care. Crit Care Med 33:1214-1224. 81. de Pablos RM, et al. (2006) Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci 26:5709-5719. 82. Crossin KL, Tai MH, Krushel LA, Mauro VP, & Edelman GM (1997) Glucocorticoid receptor pathways are involved in the inhibition of astrocyte proliferation. Proc Natl Acad Sci U S A 94:2687-2692. 83. Goujon E, Parnet P, Cremona S, & Dantzer R (1995) Endogenous glucocorticoids down regulate central effects of interleukin-1 beta on body temperature and behaviour in mice. Brain Res 702:173-180. 84. Goujon E, Parnet P, Laye S, Combe C, & Dantzer R (1996) Adrenalectomy enhances proinflammatory cytokines gene expression, in the spleen, pituitary and brain of mice in response to lipopolysaccharide. Brain Res Mol Brain Res 36:53-62. 85. Munhoz CD, et al. (2006) Chronic unpredictable stress exacerbates lipopolysaccharideinduced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci 26:3813-3820. 86. MacPherson A, Dinkel K, & Sapolsky R (2005) Glucocorticoids worsen excitotoxininduced expression of pro-inflammatory cytokines in hippocampal cultures. Exp Neurol 194:376-383. 102 87. Munhoz CD, Sorrells SF, Caso JR, Scavone C, & Sapolsky RM (2010) Glucocorticoids exacerbate lipopolysaccharide-induced signaling in the frontal cortex and hippocampus in a dose-dependent manner. J Neurosci 30:13690-13698. 88. Garcia-Fernandez LF, et al. (2000) Dexamethasone induces lipocalin-type prostaglandin D synthase gene expression in mouse neuronal cells. J Neurochem 75:460-470. 89. Uz T, et al. (1999) Glucocorticoids stimulate inflammatory 5-lipoxygenase gene expression and protein translocation in the brain. J Neurochem 73:693-699. 90. Riddick CA, Ring WL, Baker JR, Hodulik CR, & Bigby TD (1997) Dexamethasone increases expression of 5-lipoxygenase and its activating protein in human monocytes and THP-1 cells. Eur J Biochem 246:112-118. 91. Schneider N, Lanz S, Ramer R, Schaefer D, & Goppelt-Struebe M (2001) Up-regulation of cyclooxygenase-1 in neuroblastoma cell lines by retinoic acid and corticosteroids. J Neurochem 77:416-424. 92. Unlap T & Jope RS (1995) Inhibition of NFkB DNA binding activity by glucocorticoids in rat brain. Neurosci Lett 198:41-44. 93. Unlap MT & Jope RS (1997) Dexamethasone attenuates NF-kappa B DNA binding activity without inducing I kappa B levels in rat brain in vivo. Brain Res Mol Brain Res 45:83-89. 94. Wang Z, et al. (2006) The effects of dexamethasone on rat brain cortical nuclear factor kappa B (NF-kappaB) in endotoxic shock. Toxicol Appl Pharmacol 214:263-269. 95. Frank MG, Miguel ZD, Watkins LR, & Maier SF (2009) Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 96. Bowers SL, Bilbo SD, Dhabhar FS, & Nelson RJ (2008) Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun 22:105-113. 97. Sapolsky RM (1996) Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress 1:1-19. 103 98. Brewer JA, et al. (2003) T-cell glucocorticoid receptor is required to suppress COX-2mediated lethal immune activation. Nat Med 9:1318-1322. 99. Bhattacharyya S, Brown DE, Brewer JA, Vogt SK, & Muglia LJ (2007) Macrophage glucocorticoid receptors regulate Toll-like receptor 4-mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood 109:4313-4319. 100. Boyle MP, et al. (2005) Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A 102:473-478. 101. Cardona AE, Huang D, Sasse ME, & Ransohoff RM (2006) Isolation of murine microglial cells for RNA analysis or flow cytometry. Nat Protoc 1:1947-1951. 102. Cho IH, et al. (2008) Role of microglial IKKbeta in kainic acid-induced hippocampal neuronal cell death. Brain 131:3019-3033. 103. Monje ML, Toda H, & Palmer TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science 302:1760-1765. 104. Yrjanheikki J, et al. (1999) A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A 96:13496-13500. 105. Nair A & Bonneau RH (2006) Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol 171:72-85. 106. Sapolsky R, Brooke S, & Stein-Behrens B (1995) Methodologic issues in studying glucocorticoid-induced damage to neurons. J Neurosci Methods 58:1-15. 107. Stavreva DA, et al. (2009) Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol 11:1093-1102. 108. Madrigal JL, et al. (2003) Induction of cyclooxygenase-2 accounts for restraint stressinduced oxidative status in rat brain. Neuropsychopharmacology 28:1579-1588. 104 109. Garcia-Bueno B, Madrigal JL, Perez-Nievas BG, & Leza JC (2008) Stress mediators regulate brain prostaglandin synthesis and peroxisome proliferator-activated receptorgamma activation after stress in rats. Endocrinology 149:1969-1978. 110. Madrigal JL, Garcia-Bueno B, Caso JR, Perez-Nievas BG, & Leza JC (2006) Stressinduced oxidative changes in brain. CNS Neurol Disord Drug Targets 5:561-568. 111. Biber K, Neumann H, Inoue K, & Boddeke HW (2007) Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci 30:596-602. 112. Ransohoff RM & Cardona AE (The myeloid cells of the central nervous system parenchyma. Nature 468:253-262. 113. Mott RT, et al. (2004) Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia 46:369-379. 114. Hoek RM, et al. (2000) Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290:1768-1771. 115. Cardona AE, et al. (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9:917-924. 116. Nguyen KT, et al. (1998) Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci 18:2239-2246. 117. Harrison JK, et al. (1998) Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A 95:10896-10901. 118. Bhavsar PK, Sukkar MB, Khorasani N, Lee KY, & Chung KF (2008) Glucocorticoid suppression of CX3CL1 (fractalkine) by reduced gene promoter recruitment of NFkappaB. Faseb J 22:1807-1816. 119. Fong AM, et al. (1998) Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med 188:1413-1419. 105 120. Soriano SG, et al. (2002) Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J Neuroimmunol 125:59-65. 121. Roy M & Sapolsky RM (2003) The exacerbation of hippocampal excitotoxicity by glucocorticoids is not mediated by apoptosis. Neuroendocrinology 77:24-31. 122. Kroll S, et al. (2009) Control of the blood-brain barrier by glucocorticoids and the cells of the neurovascular unit. Ann N Y Acad Sci 1165:228-239. 123. Limbourg FP, et al. (2002) Rapid nontranscriptional activation of endothelial nitric oxide synthase mediates increased cerebral blood flow and stroke protection by corticosteroids. J Clin Invest 110:1729-1738. 124. Rau SW, Dubal DB, Bottner M, Gerhold LM, & Wise PM (2003) Estradiol attenuates programmed cell death after stroke-like injury. J Neurosci 23:11420-11426. 125. Miller AH (2007) Letter to the Editor Re: "an inflammatory review of glucocorticoids in the CNS" by Sorrells et al. Brain, Behavior and Immunity 21, 259-272, 2007. Brain Behav Immun 21:988-989; author reply 990. 126. Sorrells SF & Sapolsky RM (2007) An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun 21:259-272. 127. Sapolsky RM, Krey LC, & McEwen BS (1986) The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev 7:284-301. 128. Rhen T & Cidlowski JA (2005) Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med 353:1711-1723. 129. Raison CL & Miller AH (2003) When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160:1554-1565. 130. Perry VH (2007) Stress primes microglia to the presence of systemic inflammation: implications for environmental influences on the brain. Brain Behav Immun 21:45-46. 131. Stein-Behrens BA & Sapolsky RM (1992) Stress, glucocorticoids, and aging. Aging (Milano) 4:197-210. 106 132. Howard KJ & Distelhorst CW (1988) Evidence for intracellular association of the glucocorticoid receptor with the 90-kDa heat shock protein. J Biol Chem 263:3474-3481. 133. Frank MG, Baratta MV, Sprunger DB, Watkins LR, & Maier SF (2006) Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 134. Frank MG, Miguel ZD, Watkins LR, & Maier SF (2009) Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun 24:19-30. 135. Johnson JD, O'Connor KA, Watkins LR, & Maier SF (2004) The role of IL-1beta in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience 127:569-577. 136. Glezer I & Rivest S (2004) Glucocorticoids: protectors of the brain during innate immune responses. Neuroscientist 10:538-552. 137. Bruccoleri A, Pennypacker KR, & Harry GJ (1999) Effect of dexamethasone on elevated cytokine mRNA levels in chemical-induced hippocampal injury. J Neurosci Res 57:916-926. 138. Roozendaal B (2000) 1999 Curt P. Richter award. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology 25:213-238. 139. Nicholas A, Munhoz CD, Ferguson D, Campbell L, & Sapolsky R (2006) Enhancing cognition after stress with gene therapy. J Neurosci 26:11637-11643. 140. Braun S, Liebetrau W, Berning B, & Behl C (2000) Dexamethasone-enhanced sensitivity of mouse hippocampal HT22 cells for oxidative stress is associated with the suppression of nuclear factor-kappaB. Neurosci Lett 295:101-104. 141. Johnson EA, O'Callaghan JP, & Miller DB (2002) Chronic treatment with supraphysiological levels of corticosterone enhances D-MDMA-induced dopaminergic neurotoxicity in the C57BL/6J female mouse. Brain Res 933:130-138. 107 142. Limoges J, Persidsky Y, Bock P, & Gendelman HE (1997) Dexamethasone therapy worsens the neuropathology of human immunodeficiency virus type 1 encephalitis in SCID mice. J Infect Dis 175:1368-1381. 143. Roberts AJ, Crabbe JC, & Keith LD (1993) Type I corticosteroid receptors modulate PTZ-induced convulsions of withdrawal seizure prone mice. Brain Res 626:143-148. 144. Fishman RA (1982) Steroids in the treatment of brain edema. N Engl J Med 306:359-360. 145. Roberts I, et al. (2004) Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet 364:1321-1328. 146. Aisen PS, et al. (2000) A randomized controlled trial of prednisone in Alzheimer's disease. Alzheimer's Disease Cooperative Study. Neurology 54:588-593. 147. Yeager MP, et al. (2009) Pretreatment with stress cortisol enhances the human systemic inflammatory response to bacterial endotoxin. Crit Care Med 37:2727-2732. 148. Koo JW, Russo SJ, Ferguson D, Nestler EJ, & Duman RS (2010) Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A 107:2669-2674. 149. Waters JC (2009) Accuracy and precision in quantitative fluorescence microscopy. J Cell Biol 185:1135-1148. 108