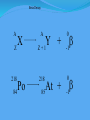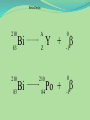* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 2, section 4 Formation of Elements
Survey
Document related concepts
Nuclear fusion wikipedia , lookup
Fallout shelter wikipedia , lookup
Gamma spectroscopy wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Nuclear fusion–fission hybrid wikipedia , lookup
Nuclear fission wikipedia , lookup
Background radiation wikipedia , lookup
Technetium-99m wikipedia , lookup
Nuclear fission product wikipedia , lookup
Ionizing radiation wikipedia , lookup
Isotopic labeling wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Radioactive decay wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Transcript
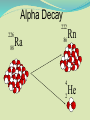
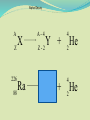
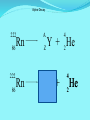
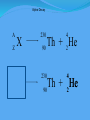
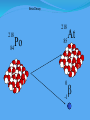
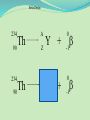
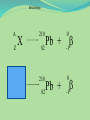
Think – Don’t Speak. What does it mean to fuse something together? When hear word fission – what does it mean? I. How Elements Form A. Nuclear Fusion – when two atomic nuclei combine 1. energy is released 2. a new element is formed Video: How are Elements Formed? www.youtube.com/watch?v=neMEo8ZrwuI II. Fusion in Stars A. Stars begin as hydrogen atoms, the H fuses to make heavier elements. B. Energy is given off in light, heat, and radiation. http://www.youtube.com/watch?v=neMEo8ZrwuI 5 min clip Alpha, Beta, and Gamma Decay The Atom The atom consists of two parts: 1. The nucleus which contains: protons neutrons 2. Orbiting electrons. The mass of an atom is almost entirely due to the number of protons and neutrons. = number of protons + Mass number number of neutrons A X Z Element symbol Atomic number = number of protons A X Z Mass # --- Atomic # #neutrons Mass # = number of protons + number of neutrons Atomic # = number of protons There are many types of uranium: 235 U 92 ISOTOPES 238 U 92 Mass # Mass # Atomic # Atomic # Number of protons Number of protons Number of neutrons Number of neutrons Number of electrons Number of electrons There are many types of uranium: 235 238 U 92 2+ U 92 Mass # 235 Mass # 238 Atomic # 92 Atomic # 92 Number of protons 92 Number of protons 92 Number of neutrons 143 Number of neutrons 146 Number of electron 92 Number of electron 90 Most of the isotopes which occur naturally are stable. A few naturally occurring isotopes and all of the man-made isotopes are unstable. Unstable isotopes can become stable by releasing different types of particles. This process is called radioactive decay and the elements which undergo this process are called radioisotopes/. Radioactive Decay Radioactive decay results in the emission of either: • an alpha particle (a), • a beta particle (b), • or a gamma ray (g). Alpha Decay An alpha particle is identical to that of a helium nucleus. It contains 2 protons and 2 neutrons. Alpha Decay 222 226 Ra 88 Rn 86 4 He 2 Alpha Decay A A-4 4 226 222 4 X Z Ra 88 Y + Z-2 Rn + 86 He 2 He 2 Alpha Decay 222 Rn 86 222 Rn 86 A 4 Y He + Z 2 218 Po + 84 4 He 2 Alpha Decay A 230 4 234 230 4 X Z U 92 Th He + 90 2 Th He + 90 2 Alpha Decay 230 Th 90 230 Th 90 A 4 226 4 Y He + Z 2 Ra He + 88 2 Beta Decay A beta particle is a fast moving electron which is emitted from the nucleus of an atom undergoing radioactive decay. Beta decay occurs when a neutron changes into a proton and an electron. Beta Decay As a result of beta decay, the nucleus has 1 less neutron, but 1extra proton. The atomic number, increases by 1 and the mass number, stays the same. Beta Decay 218 218 Po 84 At 85 b -1 0 Beta Decay A X Z 218 Po 84 A b -1 218 b -1 Y + Z+1 At + 85 0 0 Beta Decay 234 A b -1 234 234 b -1 Th 90 Th 90 Y + Z Pa + 91 0 0 Beta Decay A 210 b -1 210 210 b -1 X Z Tl 81 Pb + 82 Pb + 82 0 0 Beta Decay 210 A b -1 210 210 b -1 Bi 83 Bi 83 Y + Z Po + 84 0 0 Gamma Decay Gamma rays are not charged particles like a and b particles. Gamma rays are electromagnetic radiation with high frequency. When atoms decay by emitting a or b particles to form a new atom, the nuclei of the new atom formed may still have too much energy to be completely stable. This excess energy is emitted as gamma rays III. Synthetic Elements by Humans A. Synthetic Element – human-made, not formed in nature. B. Natural Element – forms by natural processes Synthetic Elements C. Synthetic elements are made by smashing atoms together in a particle accelerator.