* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Abstract
Survey
Document related concepts
List of types of proteins wikipedia , lookup
Magnesium transporter wikipedia , lookup
Histone acetylation and deacetylation wikipedia , lookup
Protein moonlighting wikipedia , lookup
P-type ATPase wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Homology modeling wikipedia , lookup
Protein structure prediction wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Protein domain wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Signal transduction wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Tyrosine kinase wikipedia , lookup
Phosphorylation wikipedia , lookup
Transcript
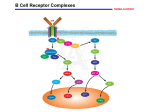
Name of Student: Shenshen Lai Research Supervisor: Dr. Steven Pelech Title of Presentation: Characterization of Phosphorylation Sites from the Activation Loop of the Mitogen-Activated Protein Kinase ERK1 Abstract Protein kinases mediate most intracellular signal transduction via the reversible phosphorylation on serine, threonine, or tyrosine residue of specific protein/peptide substrates. Such phosphorylation is employed by all eukaryotes in regulation of enzyme activity, protein-protein interaction, subcelluar localization and protease degradation. One of best studied protein kinase families is the mitogenactivated protein kinases (MAPKs). They play fundamental roles in the control of cell growth and proliferation from yeast to humans. The catalytic domains of most eukaryotic protein kinases are extremely conserved in their primary sequences, particularly in 12 functionally important kinase subdomains. The activation loop, a variable region between subdomain VII and VIII, is usually phosphorylated on multiple sites that lead to the activation of typical protein kinases. Our group has been investigating the distribution and evolutionary conservation of phosphorylation sites in 496 human protein kinase catalytic domains. All of the confirmed phosphorylation sites were mapped following an alignment of the amino acid sequences of all of these catalytic domains. About 75% of the known activatory phosphosites are located within the activation loop. From our further evolutionary analysis, phosphosites at position 154 and 157 of the alignment were identified as the most conserved sites in many human protein-serine/threonine kinases, including MAPKs, CDKs, PKCs and PKB/AKTs. In all of the MAPK members, the phosphosites corresponding to these conserved positions are located right after the well documented “TXY” activation site. The functions of these flanking sites are not clear. The three flanking phosphosites of ERK1, T198, T207 and Y210 (position 147, 154 and 157 in the kinase domain alignment, respectively) were mutated individually or in combinations, and expressed in bacteria as GST fusion proteins. The purified GST-ERKs were activated by active MEK1 in vitro and assayed against myelin basic protein (MBP) for their kinase activities. So far the results indicate regulatory roles for T207 and Y210, but not T198. By single substitution of the T207 to alanine, the activity of ERK1 towards MBP decreased by more than 50% without affecting the phosphorylation on “TEY” motif. An additional mutation at Y210 (to phenylalanine) caused the lost of most ERK1 phosphotransferase activity. However, neither of the T207E nor Y210E mutants had the ability to mimic the phosphorylation state and produce the full activity of ERK1. In addition, Y210 could be important for ERK1 to be recognized by MEK1, since the substitution of this residue to alanine but not phenylalanine blocked most of the phosphorylation on “TEY” sites by this MAPK kinase. A kinasedead (KD) mutant of ERK1 was also created by mutating K71 of subdomain II to alanine. After phosphorylation by active MEK1, a lower band shift of KD-ERK1 than the wild type was observed on the gel, which was consistent with the possibility of autophosphorylation on the three flanking phosphorylation sites. From our results, we propose that the T207 and Y210 phosphosites of ERK1 are autophosphorylated after the phosphorylation of “TEY” by MEK1, and this helps to stabilize the active state of the kinase. Our findings contribute to an improved knowledge of MAPK activation and regulation. Hyperphosphorylation within the kinase activation loop may serve as a general mechanism for protein kinases to achieve full stimulation by autophosphorylation following the initial activation by other upstream kinases.